Fig. 3.
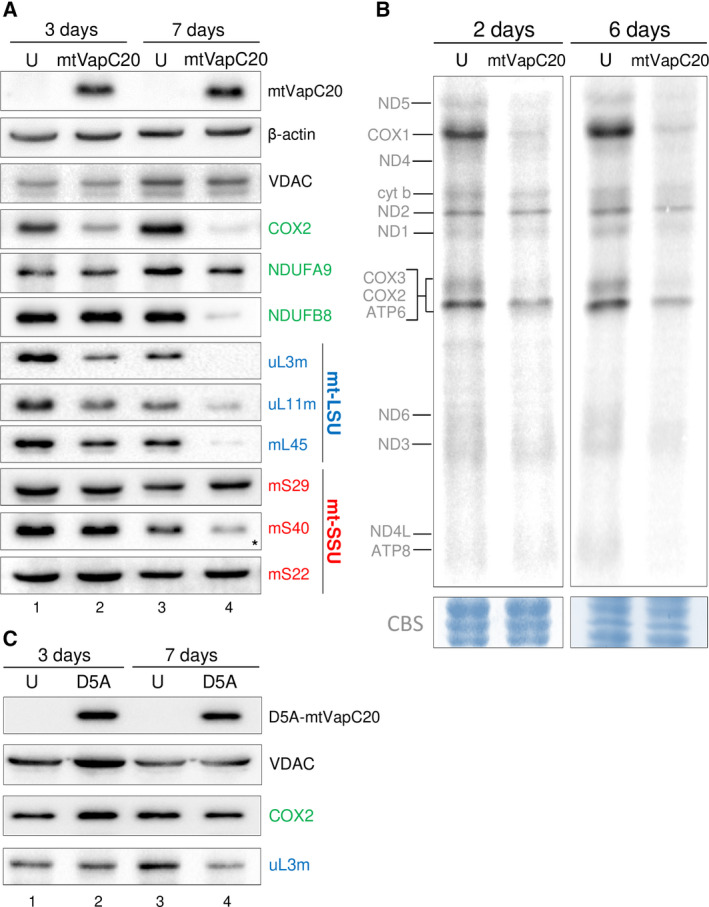
Effects of mtVapC20 expression on mitochondrial translation. (A) Cell lysates were prepared and aliquots (40 µg) subjected to immunoblotting as described. Steady‐state levels of representative OXPHOS subunits (green) were determined with antibodies against Complex I (NDUFA9 and NDUFB8) and Complex IV (COX2). Steady‐state levels of mt‐LSU (blue) and mt‐SSU (red) components were determined by western blot using antibodies against components of the mt‐LSU [HUGO protein names: MRPL3 (uL3m); MRPL11 (uL11m); MRPL45 (mL45)] and mt‐SSU [HUGO protein names: DAP3 (mS29); MRPS18B (mS40); MRPS22 (mS22)] as indicated. Asterisk indicates that the antibody possibly recognizes one of the two other mS40 isoforms, mL66 (MRPS18A), found in the mt‐LSU. Expression of mtVapC20 was detected by anti‐6XHis antibody. β‐actin and VDAC, a mitochondrial outer membrane protein, were used as loading control and mitochondrial marker, respectively. Data for each time point are representative of one experiment. (B) Metabolic 35S‐met/cys labelling of mitochondrial translation products was performed in uninduced (U) and mtVapC20 cells induced for 2 and 6 days as described. Mitochondrially encoded polypeptides were assigned after Chomyn [43]. Data for each time point are representative of one experiment. Equivalent protein loading was confirmed by Coomassie blue staining (CBS). (C) Immunoblotting analysis showing the effect of mutant mtVapC20 (D5A) expression on steady‐state levels of OXPHOS Complex IV, subunit 2 (COX2) and a constituent of the large mitoribosomal subunit (uL3m). Mutant mtVapC20 was detected with the anti‐6XHis antibody. VDAC was used as loading control. Data for each time point are representative of one experiment.
