Abstract
Background
Melanoma, a relatively common malignancy, has become one of the tumors with the fastest rising incidence in recent years. The purpose of this study was to investigate the effect of Microglial Annexin A3 (ANXA3) on melanoma.
Methods
Serum samples were obtained from 20 patients with melanoma or 20 healthy controls. Kaplan‐Meier survival analysis was performed. Transcriptome were used to analyze the correlation between ANXA3 expression and overall survival in patients with melanoma. Human melanoma cell lines WM‐115 cells were transfected with ANXA3, si‐ANXA3, ANXA3 + si‐hypoxia inducible factor‐1α (HIF‐1α), si‐ANXA3 + HIF‐1α, and negative plasmids. Cell proliferation assay, cell invasion assay, and wound healing assay were performed on WM‐115 cells. Lactate dehydrogenase (LDH) and caspase‐3/9 activities were detected by commercial kits. Western blot and RT‐PCR were used to detect the protein and mRNA expression of relation factors.
Results
ANXA3 expression was up‐regulated in patients with melanoma in comparison with healthy controls. Over‐expression of ANXA3 promoted cell growth and migration, and reduced cytotoxicity of WM‐115 cells. Overall survival (OS) and disease‐free survival (DFS) of patients with high ANXA3 expression were both lower than those of patients with low ANXA3 expression. Down‐regulation of ANXA3 reduced cell growth and migration, and promoted cytotoxicity of WM‐115 cells. ANXA3 induced vascular endothelial growth factor (VEGF) signaling pathway by activation of HIF‐1α.
Conclusion
In conclusion, our results indicated that ANXA3 promoted cell growth and migration of melanoma via activation of HIF‐1α/VEGF signaling pathway.
Keywords: ANXA3, cell growth, HIF‐1α, melanoma, migration, VEGF
ANAX3 promoted cell growth and migration of melanoma via activation of Hif‐1α/VEGF signalling pathway.
1. INTRODUCTION
Melanoma is one of the relatively common malignancies in clinical practice, and it is also one of the malignant tumors with the fastest rising incidence. 1 Although the incidence of melanoma is low in China, it has exponentially growth in recent years, with approximately 20 thousand new cases every year. Melanoma is a highly malignant skin tumor that is prone to metastasis. 2 About 50%‐80% of patients with advanced melanoma have liver metastasis, and 8% to 46% of patients with melanoma have brain metastasis. 2 The median survival time of patients with metastatic melanoma is only 8‐9 months, and the 5‐year survival rate is less than 5%. 3 Therefore, melanoma seriously endangers people's health. In recent years, rapid development has been made in drugs against melanoma, which provides better therapeutic options for most patients with melanoma. 3
Studies have shown that hypoxia can trigger a series of stress protective responses in human body. 4 , 5 Angiogenesis and increased cellular anaerobic metabolism are important ways for continuous growth and proliferation of tumor cells under hypoxic conditions and simultaneous protection from injury or death. 5 Among them, the enhanced expression and activity of hypoxia‐inducible factor‐1 (HIF‐1) is an important cause for tumor cells to adapt to the hypoxic environment. HIF‐1 is a heterodimer consisting of two subunits, HIF‐1α and HIF‐1β. HIF‐1α is the main and functional subunit of oxygen regulation. 6 HIF‐1α is rapidly degraded under normoxic conditions and stably expressed and activated during hypoxia. Therefore, it is an important regulator that connects activation of upstream and downstream in the cellular adaptation to the hypoxia pathway. 6
HIF‐1α is an oxygen‐dependent transcription factor, and hypoxic environment can promote its high expression in tumor cells. 7 Vascular endothelial growth factor (VEGF) is an important target gene of HIF‐1α, and high HIF‐1α expression can upregulate VEGF expression. 8 VEGF is currently the most potent and specific promoting factor for tumor angiogenesis. When VEGF specifically binds to endothelial cells, it can promote their proliferation, migration, and budding growth. Multiple clinical studies have shown the high expression of HIF‐1α and VEGF in malignant tumor tissues, including lung cancer, kidney cancer, pancreatic cancer, gastric cancer, colon cancer, and laryngeal cancer. 9 In addition, their expression levels are closely correlated with the clinical stage, pathological types, and prognosis of patients.
Microglial Annexin A3 (ANXA3), a member of the Annexin family, is a phospholipid and membrane‐bound protein regulated by calcium ion. 10 It is involved in membrane transport activities in cells and the response of a series of calcium‐regulated proteins on cell membrane surface, which plays an important role in inflammatory response, cell growth and differentiation, and cytoskeletal protein response. The abnormal ANXA3 expression is associated with several diseases. 11 Moreover, it can mediate cell proliferation, apoptosis, motility, and signal transduction, thereby playing an important role in tumorigenesis and tumor development. 12 , 13
2. MATERIALS AND METHODS
2.1. Clinical patients with melanoma and microarray
Serum samples were obtained from 20 patients with melanoma or 20 normal volunteers in Zhejiang Rehabilitation Medical Center (Hangzhou, China). Written informed consent was obtained from all participants, and the research protocols were approved by the Ethics Committee of Zhejiang Rehabilitation Medical Center. Microarray (ME1004) was purchased from Alenabio.
2.2. Cell culture and small‐interfering RNA transfection
Human melanoma cell lines or WM‐115 cells were purchased from Shanghai Cell bank and maintained in RPMI‐1640 medium (Hyclone) containing 10% fetal bovine serum (FBS; Hyclone) at 37°C with 5% CO2. ANXA3, si‐ANXA3, ANXA3 + siHIF‐1α, si‐ANXA3 + HIF‐1α, and negative plasmids were synthesized by GenePharm and transfected using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer's protocol.
2.3. Quantitative RT‐PCR
Total RNA was isolated from human serum samples or WM‐115 cells using Trizol reagent (Thermo Fisher Scientific), and the RNA purity was detected using spectrophotometer. The synthesis of cDNA was performed by the First Strand cDNA Synthesis Kit (Fermentas; Thermo Fisher Scientific). Quantitative RT‐PCR was carried out by ABI 7500 Fast Real‐Time PCR system (Applied Biosystems) with SYBRGreen PCR Kit (Takara). These reactions were incubated at 95°C for 5 minutes, followed by 40 cycles of 95°C for 30 seconds, 60°C for 40 seconds, and 72°C for 30 seconds. Relative quantitation of the gene expression was normalized by β‐actin following the 2‐ΔΔCt method and relative to the control group.
2.4. Western blot
Cells and serum samples were lysed with RIPA buffer (Beyotime Institute of Biotechnology, Shanghai, China), and the protein concentration was determined by BCA protein concentration assay kit (Beyotime Institute of Biotechnology, Shanghai, China). Equal amounts of protein samples (50 μg) were separated by SD‐PAGE (BIO‐RAD Co.) and then transferred to PVDF membrane. The membrane was incubated with the primary antibodies (Abcam Technology), including anti‐ANXA3, anti‐HIF‐1α, anti‐VEGF, anti‐GAPDH, and anti‐β‐Actin, overnight at 4°C and then with secondary antibodies conjugated to horseradish peroxidase (ZSGB Biotech Co., Ltd.) for 1 hour at room temperature. Protein blank was visualized by enhanced chemiluminescence (ECL) reagent (Tsea biotech Co.) and calculated with Image‐ProPlus 6.0 software (Media Cybernetics, Inc.).
2.5. Cell proliferation assay and cell invasion assay
Cells were incubated with MTT (Sigma‐Aldrich) for 4 hours, and 200 μL of DMSO was added to each well for 0.5 hour at 37°C. The absorbance was evaluated at 492 nm using a microplate reader (Bio‐Rad Laboratories Inc.).
Cells were plated on the upper Transwell chambers (Costar; Corning Incorporated) at 24‐well Transwell culture chamber and 500 mL of RPMI‐1640 medium supplemented with 10% FBS was added to the lower compartment. After 48 hours of incubation, cells were fixed with methanol for 1 hour and traversed cells on the lower side of the filter were stained with crystal violet and counted.
2.6. Wound healing assay
Cells were plated at 24‐well Transwell and cultured until 90% confluence. 200‐μL pipette tip was made for artificial wound in the center of the confluent cell monolayer, and cells were cultured for 24 hours. The closure of the wound in each group was evaluated under microscope.
2.7. Lactate dehydrogenase activity assay and caspase‐3/9 activity assay
Lactate dehydrogenase (LDH) or Caspase‐3/9 activity levels were measured using LDH activity kit or Caspase‐3/9 activity kits. The absorbance was evaluated at 492 or 405 nm using a microplate reader (Bio‐Rad Laboratories Inc.).
2.8. Survival analysis of melanoma patients
Kaplan–Meier survival analysis was performed by GraphPad. Melanoma transcriptome were utilized to analyze the correlation between ANXA3 expression and overall survival (OS) in melanoma patients.
2.9. Statistical methods
Data are expressed as means ± standard deviation (SD) using GraphPad Prism (GraphPad Software, San Diego, CA, USA). Two‐way repeated measures ANOVA with Tukey's post hoc test or Student's t tests was used to compare the differences between groups. A value of P < .05 was considered to be significant.
3. RESULTS
3.1. The serum ANXA3 levels in patients with melanoma
To investigate the biological functions of ANXA3 in patients with melanoma, we evaluated potential targets of melanoma. As shown in Figure 1A,B, the serum ANXA3 levels in patients with melanoma were up‐regulated in comparison with healthy controls. 3D construction of ANXA3 was showed in Figure 1C. Along with increased pathological grade, the serum ANXA3 expression levels in patients with melanoma of 3 or 4 grade were up‐regulated in comparison with patients with 1 or 2 grade (Figure 1D). OS of patients with low ANXA expression was higher than that of patients with high ANXA expression (Figure 1E). DFS of patients with low ANXA expression was similarity with patients with high ANXA expression (Figure 1F).
Figure 1.
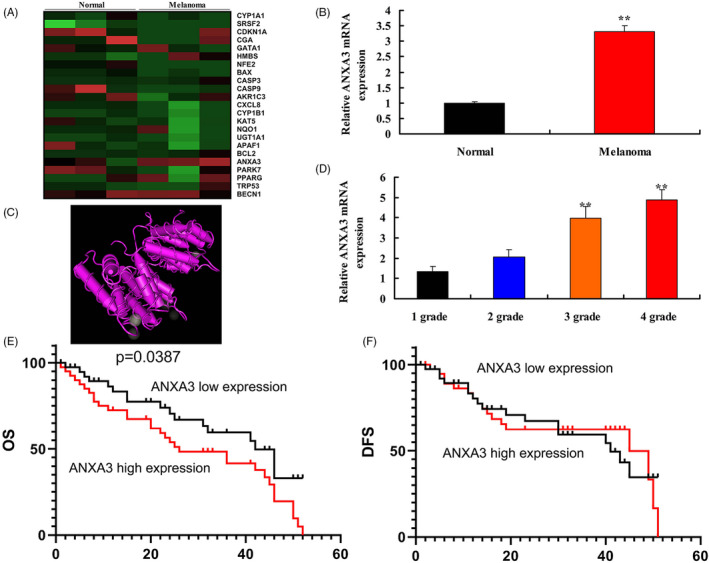
The serum ANXA3 levels in patients with melanoma. Heat map (A), serum ANXA3 mRNA levels (B), 3D construction of ANXA3 (C), pathological grade and serum levels of ANXA (D), OS and DFS of patients with low ANXA expression was higher than that of patients with high ANXA expression (E and F). Normal, normal volunteer group; Melanoma, melanoma patients group; 1 grade, 1 grade melanoma patients group; 2 grade, 2 grade melanoma patients group; 3 grade, 3 grade melanoma patients group; 4 grade, 4 grade melanoma patients group. **P < .01 versus normal volunteer group or 1 grade melanoma patients group
3.2. ANXA3 regulated cell growth of WM‐115 cells
ANXA3 plasmid (negative, 25, 50 and 100 ng) were transfected into WM‐115 cells and increased ANXA3 expressions levels in vitro model (Figure 2A,B). Over‐expression of ANXA3 promoted cell growth of WM‐115 cells (Figure 2C). Meanwhile, siANXA3 plasmid (negative, 25, 50, and 100 ng) were transfected into WM‐115 cells and decreased ANXA3 expressions levels in vitro model (Figure 2D,E). Down‐regulation of ANXA3 reduced cell growth of WM‐115 cells (Figure 2F).
Figure 2.
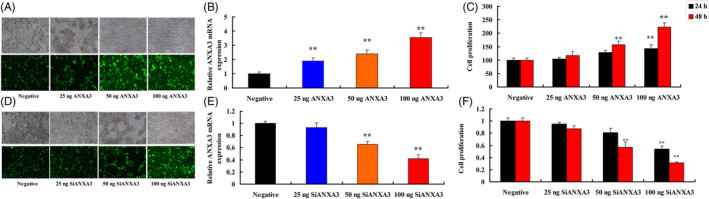
ANXA3 regulated cell growth of WM‐115 cells. Fluorescence for plasmid (A), ANXA3 mRNA expression (B), cell growth (C) by over‐expression of ANXA3; fluorescence for plasmid (D), ANXA3 mRNA expression (E), cell growth (F) by down‐regulation of ANXA3. Negative, negative group; 25 ng ANXA3, 25 ng of ANXA3 group; 50 ng ANXA3, 50 ng of ANXA3 group; 100 ng ANXA3, 100 ng of ANXA3 group; 25 ng siANXA3, 25 ng of siANXA3 group; 50 ng siANXA3, 50 ng of siANXA3 group; 100 ng siANXA3, 100 ng of siANXA3 group. **P < .01 versus negative group
3.3. HIF‐1α/VEGF signaling pathway was important signaling pathway in the pro‐cancer effects of ANXA3 on melanoma
Heat map of gene chip showed that ANXA3 induced HIF‐1α/VEGF signaling pathway (Figure 3A). Heat map of ANXA3 regulated other signaling pathways was showed in Figure 3B. HIF‐1α/VEGF signaling pathway maybe an important signaling pathway in the pro‐cancer effects of ANXA3 on melanoma (Figure 3C). Over‐expression of ANXA3 induced the protein expressions levels of ANXA3, HIF‐1α, and VEGF in WM‐115 cells (Figure 3D,F,H). Down‐regulation of ANXA3 suppressed the protein expressions levels of ANXA3, HIF‐1α, and VEGF in WM‐115 cells (Figure 3E,I,K).
Figure 3.
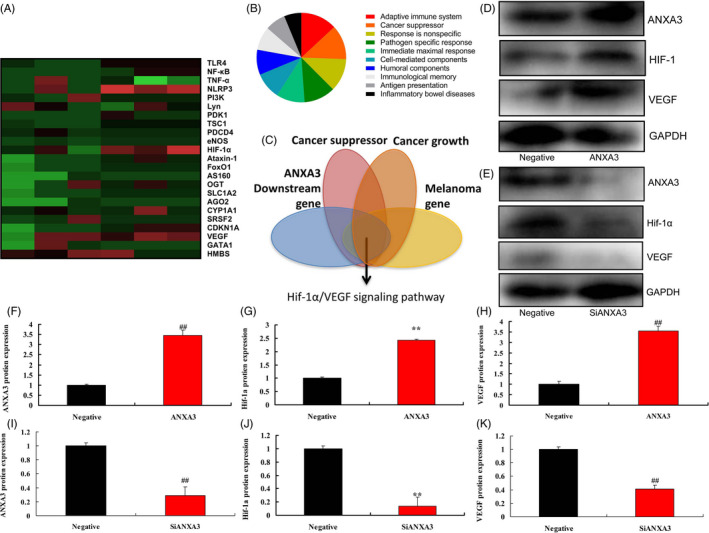
HIF‐1α/VEGF signaling pathway is important signaling pathway in the pro‐cancer effects of ANXA3 on melanoma. Heat map (A), Scale analysis diagram (B), refine results (C); Western blot for ANXA3/HIF‐1α/VEGF by over‐expression of ANXA3 (D) and ANXA3/HIF‐1α/VEGF by down‐regulation of ANXA3 (E), ANXA3/HIF‐1α/VEGF expression by over‐expression of ANXA3 (F‐H), ANXA3/HIF‐1α/VEGF expression by down‐regulation of ANXA3 (I‐K). Negative, negative group; ANXA3, 50 ng of ANXA3 group; siANXA3, 50 ng of siANXA3 group. **P < .01 versus negative group
3.4. ANXA3 regulated cell progression and migration of WM‐115 cells
Over‐expression of ANXA3 promoted cell progression and migration, and reduced LDH activity and caspase‐3/9 activity levels in WM‐115 cells (Figure 4A‐G). Down‐regulation of ANXA3 reduced cell progression and migration, and increased LDH activity and caspase‐3/9 activity levels in WM‐115 cells (Figure 4H‐N).
Figure 4.
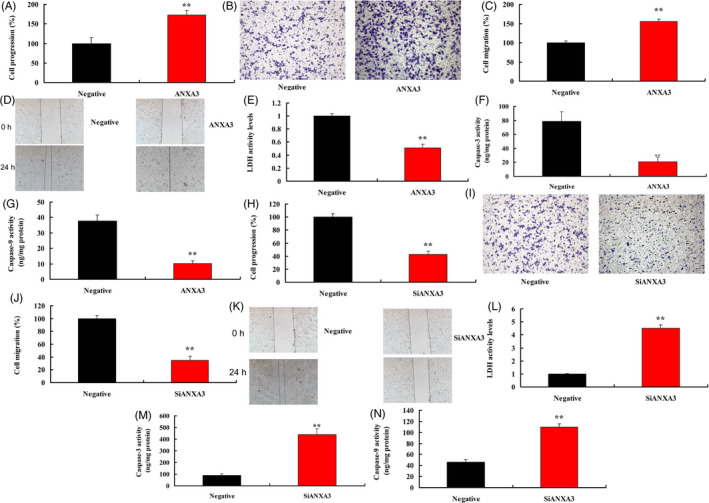
ANXA3 regulated cell progression and migration of WM‐115 cells. Cell progression (A and B), cell migration (C and D), LDH activity, and caspase‐3/9 activity levels (E‐G) by over‐expression of ANXA3; cell progression (H and I), cell migration (J and K), LDH activity and caspase‐3/9 activity levels (L‐N) by down‐expression of ANXA3; Negative, negative group; ANXA3, 50 ng of ANXA3 group; siANXA3, 50 ng of siANXA3 group. **P < .01 versus negative group
3.5. The regulation of HIF‐1α in the pro‐cancer effects of ANXA3 in WM‐115 cells
The experiment further investigated the role of HIF‐1α in the pro‐cancer effects of ANXA3 on melanoma. Down‐regulation of HIF‐1α reduced cell growth, cell progression and migration, and promoted LDH activity and caspase‐3/9 activity levels in WM‐115 cells by over‐expression of ANXA3 (Figure 5A‐H). Down‐regulation of HIF‐1α also suppressed the protein expressions levels of HIF‐1α and VEGF in WM‐115 cells by over‐expression of ANXA3 (Figure 5I‐K). Up‐regulation of HIF‐1α promoted cell growth, cell progression and migration, and reduced LDH activity and caspase‐3/9 activity levels in WM‐115 cells by down‐regulation of ANXA3 (Figure 6A‐H). Up‐regulation of HIF‐1α also induced the protein expressions levels of HIF‐1α and VEGF in WM‐115 cells by over‐expression of ANXA3 (Figure 6I‐K).
Figure 5.
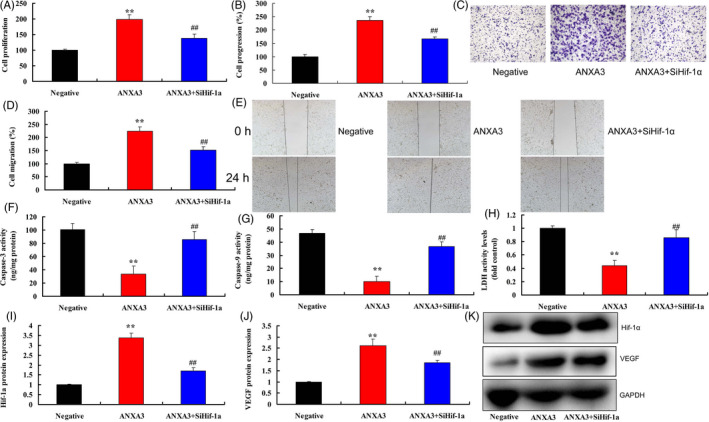
The inhibition of HIF‐1α in the pro‐cancer effects of ANXA3 on melanoma. Cell growth (A), cell progression (B and C), cell migration (D and E), LDH activity and caspase‐3/9 activity levels (F‐H), HIF‐1α/VEGF expression (I‐K). Negative, negative group; ANXA3, 50 ng of ANXA3 group; ANXA3 + siHif‐1α, 50 ng of ANXA3 group and down‐regulation of Hif‐1α. **P < .01 versus negative group, *P < .01 versus 50 ng of ANXA3
Figure 6.
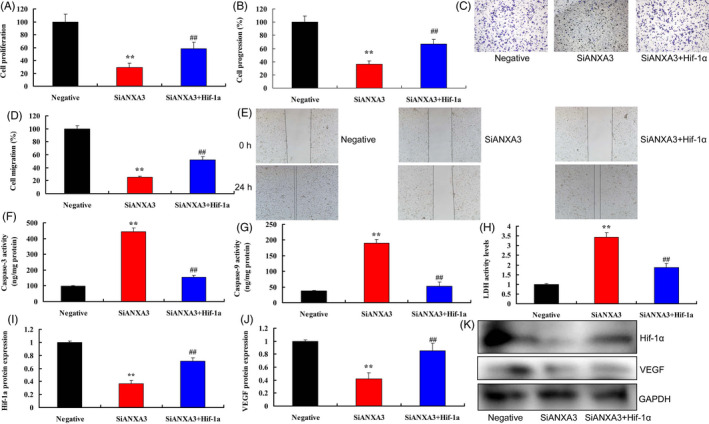
The induction of HIF‐1α in the pro‐cancer effects of ANXA3 on melanoma. Cell growth (A), cell progression (B and C), cell migration (D and E), LDH activity and caspase‐3/9 activity levels (F‐H), HIF‐1α/VEGF expression (I‐K). Negative, negative group; si‐ANXA3, 50 ng of siANXA3 group; si‐ANXA3 + HIF‐1α, 50 ng of si‐ANXA3 group, and up‐regulation of HIF‐1α. **P < .01 versus negative group, *P < .01 versus 50 ng of ANXA3
4. DISCUSSION
Melanoma, a relatively common malignant tumor, has become one of the tumors with the fastest rising incidence in recent years. 14 According to statistics, there were 232 thousand new cases of melanoma and 55 thousand cases of death worldwide in 2012. 15 , 16 In contrast, the proportion of death case and new case of melanoma in China is significantly higher than the global level. 15 , 16 In our study, the serum ANXA3 levels in patients with melanoma were up‐regulated. Over‐expression of ANXA3 promoted cell growth, cell progression, and migration of WM‐115 cells. Wang et al showed that depletion of ANXA3 suppressed cell proliferation and tumor growth of gastric cancer. 17 These results showed that ANXA3 promoted cell growth in melanoma and may induce the development of melanoma.
HIF‐1α is a transcription factor produced by tissues during ischemia and hypoxia, which is involved in growth, infiltration, and metastasis of various tumor cells. 18 Studies have found that HIF‐1α downstream contains more than one hundred types of target genes. 19 , 20 The products of HIF‐1α target genes play an important role in erythropoiesis, angiogenesis, cell proliferation, differentiation and apoptosis, and infiltration and metastasis of tumor cells. 18 Here, our results showed that Over‐expression of ANXA3 induced the protein expressions levels of HIF‐1α and VEGF in WM‐115 cells. HIF‐1α/VEGF signaling pathway is important signaling pathway in the pro‐cancer effects of ANXA3 on melanoma.
In addition, HIF‐1α can also participate in promoting angiogenesis by up‐regulating the expression of VEGF. 21 The role of VEGF in the occurrence and development of breast cancer, glioma, renal cell carcinoma, lung cancer, liver cancer, and colon cancer has also been confirmed. 22 The expression levels of HIF‐1α and VEGF are significantly higher in the hypoxic environment than those in the normoxic environment, indicating that the hypoxic environment can promote the VEGF expression by up‐regulating HIF‐1α expression. 23 Malignant melanoma has high potential for proliferation, local infiltration, and distant metastasis, which is associated with the high VEGF expression. 23 VEGF can induce tumor angiogenesis and accelerate the invasion and metastasis of malignant melanoma. Interestingly, our results showed that the down‐regulation of HIF‐1α reduced the pro‐cancer effects of ANXA3 on cell growth in melanoma. Zhang et al reported that the down‐regulation of ANXA3 alleviated bone cancer via inhibiting HIF‐1α/VEGF signaling pathway. 24 Taken together, these results showed that HIF‐1α/VEGF signaling pathway may be related to the regulation of ANXA3 in melanoma.
In conclusion, ANXA3 plays a key role in pro‐cancer effects of melanoma by inducing HIF1α/VEGF signaling pathway. Therefore, the study suggests that ANXA3 may be a potential therapeutic target for melanoma.
ACKNOWLEDGMENTS
This work was supported by Natural Science Foundation of Zhejiang Province of China (LY16H150006) and Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2018247152).
Xu B, Zhang X, Gao Y, Song J, Shi B. Microglial Annexin A3 promoted the development of melanoma via activation of hypoxia‐inducible factor‐1α/vascular endothelial growth factor signaling pathway. J Clin Lab Anal.2021;35:e23622 10.1002/jcla.23622
REFERENCES
- 1. Pelczar P, Witkowski M, Perez LG, et al. A pathogenic role for T cell‐derived IL‐22BP in inflammatory bowel disease. Science. 2016;354(6310):358‐362. [DOI] [PubMed] [Google Scholar]
- 2. Morampudi V, Dalwadi U, Bhinder G, et al. The goblet cell‐derived mediator RELM‐β drives spontaneous colitis in Muc2‐deficient mice by promoting commensal microbial dysbiosis. Mucosal Immunol. 2016;9(5):1218‐1233. [DOI] [PubMed] [Google Scholar]
- 3. Yang GX, Sun Y, Tsuneyama K, et al. Endogenous interleukin‐22 protects against inflammatory bowel disease but not autoimmune cholangitis in dominant negative form of transforming growth factor beta receptor type II mice. Clin Exp Immunol. 2016;185(2):154‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li L, Hai J, Li Z, et al. Resveratrol modulates autophagy and NF‐κB activity in a murine model for treating non‐alcoholic fatty liver disease. Food Chem Toxicol. 2014;63:166‐173. [DOI] [PubMed] [Google Scholar]
- 5. Kim W, Kim BG, Lee JS, et al. Randomised clinical trial: the efficacy and safety of oltipraz, a liver X receptor alpha‐inhibitory dithiolethione in patients with non‐alcoholic fatty liver disease. Aliment Pharmacol Ther. 2017;45(8):1073‐1083. [DOI] [PubMed] [Google Scholar]
- 6. Shankar E, Zhang A, Franco D, Gupta S. Betulinic acid‐mediated apoptosis in human prostate cancer cells involves p53 and nuclear factor‐kappa B (NF‐κB) pathways. Molecules. 2017;22(2):264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou Y, Ding YL, Zhang JL, Zhang P, Wang JQ, Li ZH. Alpinetin improved high fat diet‐induced non‐alcoholic fatty liver disease (NAFLD) through improving oxidative stress, inflammatory response and lipid metabolism. Biomed Pharmacother. 2018;97:1397‐1408. [DOI] [PubMed] [Google Scholar]
- 8. Sharifi N, Amani R, Hajiani E, Cheraghian B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non‐alcoholic fatty liver disease? A randomized clinical trial. Endocrine. 2014;47(1):70‐80. [DOI] [PubMed] [Google Scholar]
- 9. Mukai T, Egawa M, Takeuchi T, Yamashita H, Kusudo T. Silencing of FABP1 ameliorates hepatic steatosis, inflammation, and oxidative stress in mice with nonalcoholic fatty liver disease. FEBS Open Bio. 2017;7(7):1009‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahmad A, Ginnebaugh KR, Sethi S, et al. miR‐20b is up‐regulated in brain metastases from primary breast cancers. Oncotarget. 2015;6(14):12188‐12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao S, Li T, Li J, et al. miR‐23b‐3p induces the cellular metabolic memory of high glucose in diabetic retinopathy through a SIRT1‐dependent signalling pathway. Diabetologia. 2016;59(3):644‐654. [DOI] [PubMed] [Google Scholar]
- 12. Huang H, Hu M, Zhao R, Li P, Li M. Dihydromyricetin suppresses the proliferation of hepatocellular carcinoma cells by inducing G2/M arrest through the Chk1/Chk2/Cdc25C pathway. Oncol Rep. 2013;30(5):2467‐2475. [DOI] [PubMed] [Google Scholar]
- 13. Rotman Y. Similarity between studies of dihydromyricetin and reservatrol for NAFLD. Pharmacol Res. 2015;100:335. [DOI] [PubMed] [Google Scholar]
- 14. Mizoguchi A, Yano A, Himuro H, Ezaki Y, Sadanaga T, Mizoguchi E. Clinical importance of IL‐22 cascade in IBD. J Gastroenterol. 2018;53(4):465‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bootz F, Ziffels B, Neri D. Antibody‐based targeted delivery of interleukin‐22 promotes rapid clinical recovery in mice with DSS‐induced colitis. Inflamm Bowel Dis. 2016;22(9):2098‐2105. [DOI] [PubMed] [Google Scholar]
- 16. Hartmann P, Seebauer CT, Mazagova M, et al. Deficiency of intestinal mucin‐2 protects mice from diet‐induced fatty liver disease and obesity. Am J Physiol Gastrointest Liver Physiol. 2016;310(5):G310‐G322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coskun M, Bjerrum JT, Seidelin JB, Troelsen JT, Olsen J, Nielsen OH. miR‐20b, miR‐98, miR‐125b‐1*, and let‐7e* as new potential diagnostic biomarkers in ulcerative colitis. World J Gastroenterol. 2013;19:4289‐4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirayama Y, Koizumi S. Hypoxia‐independent mechanisms of HIF‐1α expression in astrocytes after ischemic preconditioning. Glia. 2017;65(3):523‐530. [DOI] [PubMed] [Google Scholar]
- 19. Wang G, Wang JJ, Fu XL, Guang R, To ST. Advances in the targeting of HIF‐1α and future therapeutic strategies for glioblastoma multiforme. Oncol Rep. 2017;37(2):657‐670. [DOI] [PubMed] [Google Scholar]
- 20. Houghton D, Thoma C, Hallsworth K, et al. Exercise reduces liver lipids and visceral adiposity in patients with nonalcoholic steatohepatitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2017;15(1):96‐102.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen Y, Lv L, Pi H, et al. Dihydromyricetin protects against liver ischemia/reperfusion induced apoptosis via activation of FOXO3a‐mediated autophagy. Oncotarget. 2016;7(47):76508‐76522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Song Q, Liu L, Yu J, et al. Dihydromyricetin attenuated Ang II induced cardiac fibroblasts proliferation related to inhibitory of oxidative stress. Eur J Pharmacol. 2017;807:159‐167. [DOI] [PubMed] [Google Scholar]
- 23. Zhou DZ, Sun HY, Yue JQ, Peng Y, Chen YM, Zhong ZJ. Dihydromyricetin induces apoptosis and cytoprotective autophagy through ROS‐NF‐kappaB signalling in human melanoma cells. Free Radical Res. 2017;51:517‐528. [DOI] [PubMed] [Google Scholar]
- 24. Ma H, Guo S, Luo Y, et al. MicroRNA‐20b promotes the accumulation of CD11b+Ly6G+Ly6Clow myeloid‐derived suppressor cells in asthmatic mice. Cent Eur J Immunol. 2017;42(1):30‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]


