Abstract
In this grant report, we describe our project to expand measurement-based psychiatric care across 6 early psychosis treatment teams in Minnesota, and to provide a neuroscience-informed cognitive training and motivation enhancement program for individuals with early psychosis. This project is part of the NIMH Early Psychosis Intervention Network (EPINET) initiative which seeks to link data from treatment centers nationally that offer evidence-based specialty care to persons experiencing early psychosis. Systematic analyses of pooled data collected in EPINET will help inform methods for early psychosis care, psychosis risk factors, and pre-emptive interventions. As part of the national EPINET, our hub (Early Psychosis Intervention-Minnesota, EPI-MINN), will: (1) provide measurement-based care in coordinated specialty care programs for early psychosis, (2) determine whether a structured feedback report provides benefit to stakeholders—service users, family members, and primary clinicians, and (3) explore whether deficits in cognition and motivated behavior—two domains that significantly impact functioning and overall quality of life in early psychosis—can be addressed as key treatment goals by implementing a 12-week mobile intervention. Using a regression discontinuity design, participants will be randomized to the cognitive training and motivational enhancement intervention or to treatment as usual. The intervention consists of neuroscience-informed, computerized auditory and social cognitive training exercises, as well as a mobile app where participants interact with each other and with a motivational coach. Participants will complete assessments at 4 time points: baseline and post-intervention (i.e., at 6 months), and again at 12 and 18 months to test the long-term effects of the intervention. All assessments and interventions in this project can be completed entirely remotely.
Keywords: measurement-based care, cognitive training, first episode psychosis
INTRODUCTION
Schizophrenia and related psychotic disorders are low prevalence disorders with a high global disease burden. In 2016, global prevalence was found to be 0.28%. Over 70% of those with schizophrenia were in the age group of 25–54. As people with schizophrenia are more likely to be unemployed and more dependent on others for daily living support, the burden is large as this is an age group that is generally the most economically productive [1]. Despite the low prevalence, treatment needs are often not being met. In a meta-analysis investigating recovery rates, only 13.5% of patients with schizophrenia and related psychoses met criteria for recovery (measured with both clinical and social scales) [2]. Early intervention has shown to be crucial in psychotic illnesses, and coordinated specialty care programs have been developed to provide comprehensive early intervention services. Coordinated specialty care programs include services such as psychotherapy, medication management, psychoeducation, and work or education support.
Coordinated specialty care programs, such as the Recovery after an Initial Schizophrenia Episode (RAISE) Early Treatment Program (ETP), represent an exciting new evidence-based intervention for psychosis spectrum disorders [3]. However, RAISE-ETP benefits in quality of life were seen primarily during the first 6 months of treatment, and only for individuals whose duration of untreated psychosis was <74 weeks. Thus, a key scientific question from the RAISE study is: Can coordinated specialty care be enhanced in order to generate greater benefits for more individuals?
SPECIFIC AIMS
The purpose of this project is to expand measurement-based psychiatric care across 6 early psychosis treatment teams, distributed across 4 different clinical sites in Minnesota, each providing coordinated specialty care and in total serving approximately 200 individuals per year. Our first goal is to efficiently deploy valid longitudinal outcome measures across each team, implement state-of-the-art informatics tools, and aggregate pooled data to inform and support program evaluation activities as well as novel data-driven analytics. Our approach will be enhanced by use of a central assessment team to provide highly reliable remote diagnostic evaluations across all sites. Second, we propose a practice-based research project that seeks to answer two questions: (1) Does a structured personalized feedback session that includes an explicit focus on cognition and motivated behavior provide benefit to stakeholders—service users, family members, and primary clinicians? (2) Can cognition and motivated behavior be addressed as key treatment goals within real-world settings, using a 12-week mobile intervention program?
Targeting cognitive impairment early in treatment could improve outcomes, particularly for more impaired individuals. We have previously shown that auditory cognitive training can be successfully delivered on a mobile device to individuals with early schizophrenia, resulting in significant gains in global cognition and lower symptoms that endure 6 months after the intervention [4,5]. We have found that adding social cognitive training exercises further improves measures of motivated behavior [6].
A strong body of evidence also indicates that motivational impairment is a determining factor for quality of life and functional outcome in early psychosis. We recently showed that a 12-week mobile digital health coaching and social networking app designed to target motivated behavior in early psychosis resulted in significantly greater improvements in self-reported depression, defeatist beliefs, self-efficacy, and a trend towards improved motivation/pleasure and negative symptoms (compared to a wait-list control) [7]. These improvements were maintained when reassessed 3 months after the end of the trial.
Therefore, our central scientific premise is to investigate the potential benefits of targeting cognitive functioning and motivation in people with early psychosis, using a well-defined 12-week mobile intervention program that combines cognitive training with a coaching and social networking app.
Aim 1: Establish Highly Reliable Measurement-Based Psychiatric Care for 200 Early Psychosis Individuals per Year across 6 Clinical Teams; Harness Data for Novel Predictive Analyses
Partner with six coordinated specialty care teams in Minnesota to implement standardized clinical assessments, timely feedback to clinicians about patients’ progress, and systematic monitoring of key outcomes. Establish the informatics platform to collect, aggregate, and manipulate clinical encounter data to support data-guided fidelity monitoring and quality improvement. Interface with EPINET National Coordinating Data Center.
Use aggregated clinical encounter data to perform novel data-driven trajectory analyses, predictive modeling, and causal discovery analyses to uncover potential determinants of outcome that might suggest treatment program refinements and/or personalized courses of treatment.
Aim 2: Investigate Potential Benefits of Identifying Cognitive Functioning and Motivated Behavior as Explicit Treatment Targets in Early Psychosis; Study a Well-Defined 12-Week Mobile Intervention Program to Address These Targets
Implement brief, reliable, digitally-supported measures of cognition and motivated behavior as part of the clinical assessment process. Determine the benefit to stakeholders of providing measurement-based personalized feedback about cognition and motivation as part of the evaluation and treatment-planning process.
Investigate the six-month, twelve-month and eighteen-month effects post-intervention of a 12 week mobile cognitive training program that includes social cognition training, combined with an evidence-based mobile app targeting motivation in early psychosis, using a cut-off based design (variant of regression discontinuity design).
SIGNIFICANCE
Aim 1A: Measurement-Based Psychiatric Care Results in Enhanced Outcomes
The goal of AIM 1A is to establish a coordinated and integrated approach to measurement-based care across 6 early psychosis treatment teams taking place across 4 different clinical sites. Measurement-based care (MBC) is a strategy to support evidence-based psychiatric treatment [8]. A key feature of evidence-based treatment is continuous assessment and feedback to patients to understand how symptoms and functioning are responding to treatment strategies. As well as assessing fidelity to maximize treatment outcomes and providing staff supervision to support and hone clinical effectiveness. MBC in psychiatric settings results in increased satisfaction with care, improvements in therapeutic outcomes, and decreases in symptom severity [9–11]. MBC is furthermore associated with improvements in clinical effectiveness and improved coordination and collaboration between individuals and their treatment providers [11]. Reductions in health care costs seen with MBC often are linked to an increased ability to evaluate quality improvement [9,12,13].
Deploying MBC in real-world settings has primarily focused on symptoms, but it can also be used to assess functioning, mechanisms of change, and the treatment process itself (session feedback, therapeutic alliance, etc.) [13]. MBC along with measurement-based feedback are key drivers of precision mental health, but it is vital that there are standard measures with established reliability and validity [14]. Clinicians involved in MBC deliver a practice-based evidence approach aided by regular and consistent assessments with patients; the feedback delivered to patients and clinicians results in a better shared understanding of individualized treatment and modifications to enhance patient outcomes [8]. Utilizing MBC allows providers to be more precise through regular targeted assessments of key clinical outcomes [11]. The benefits of MBC are maximized and sustained when there is simultaneous monitoring of treatment and fidelity outcomes along with timely feedback to service providers and patients [8,13]. MBC and MBF have multiple uses in real-world settings including clinical feedback, supporting supervision, and assisting in data-driven decision making for administrators and agencies [8].
Aim 1B: Longitudinal Measurement-Based Clinical Data Sets Can Support Innovative Data-Driven Predictive Analyses That Uncover Treatment Response Patterns and Support Precision Psychiatry
The goal of AIM 1B is to use the data acquired in AIM 1A, not just for clinician feedback and program monitoring, but for innovative data-driven predictive analyses that deepen our understanding of treatment response patterns and can potentially inform new treatment approaches.
Latent Class Growth Analysis (LCGA) [15–18] is an analytic approach that allows one to characterize the heterogeneous dynamic response to treatment——a key feature of psychiatric disorders. This data-driven computational method identifies subgroups (latent classes) of patients that share similar response patterns or trajectories over time. Potential response patterns include rapid recovery, slow recovery, rapid deterioration, slow deterioration. These response patterns have been shown to be effective in capturing the different patterns of progression of complex diseases [19–21]. After the identification of the different response patterns, one can then test hypotheses about whether specific baseline variables or a specific treatment has a significant effect on response patterns. Since patients are assigned into trajectory subgroups according to their response patterns rather than according to their characteristics or the specific treatment they receive, this approach captures the dynamics of the heterogeneous response patterns seen within treatment groups. In Aim 1B, we will use LGCA to examine the longitudinal data obtained in Aim 1A.
Furthermore, identifying variables that are indicative of patterns in the response to treatment can inform the development of novel personalized treatment strategies. Supervised learning predictive modeling techniques [21–24] permit one to identify such variables and are especially well-suited to tackle the challenges in psychiatric datasets, including various distributional patterns among variables, high dimensional data relative to sample size (e.g., many hundreds of variables were collected in RAISE ETP), and complex interactions among measured variables. The application of such machine learning techniques in psychiatry has recently resulted in promising diagnostic and prognostic “precision psychiatry” models for various disorders [19,25–28], and will be also used in support of Aim 1B.
Aim 2A: Cognitive Dysfunction and Impaired Motivation Are Critical Treatment Targets in Early Psychosis
The goal of Aim 2A is to implement—as part of the measurement-based care provided in Aim 1A—a brief set of reliable, digitally-supported measures of cognition and motivated behavior as part of the clinical assessment process. We will determine whether stakeholders (clients, caregivers, and primary clinicians) find it helpful to receive measurement-based personalized feedback about cognition and motivation as part of the evaluation and treatment-planning process. Feedback will focus on strengths and areas for improvement.
Based on our pilot work in clinical settings, we posit that participatory decision-making is strongly facilitated when stakeholders have full access to measurement-based personalized profiles that help them identify their own individual strengths and areas for growth that can be followed and assessed over time. We posit that measures of cognition and motivated behavior should be an integral part of those profiles and provide a high degree of useful information to stakeholders.
Cognitive dysfunction is a core pathophysiological feature of psychosis and one of the strongest predictors of functional outcomes [29–31]. Several studies indicate that current early intervention programs may not significantly alter long-term clinical outcome, suggesting that critical treatment target(s), beyond symptoms and functional status, are not being addressed [32,33]. Evidence strongly indicates that, along with cognitive dysfunction, impaired motivation is also a critical target and unmet therapeutic need [34–39].
Aim 2B: Cognitive Dysfunction and Impaired Motivation Improve with Scalable Mobile Interventions
The goal of Aim 2B is to follow up on the cognitive and motivation profiles identified in AIM 2A, and to investigate the six- and twelve-month effects of 12 weeks of mobile cognitive training combined with an evidence-based mobile app targeting motivation in early psychosis. We base this goal on our extensive multi-site research experience with these interventions, both within community mental health centers and delivered entirely remotely (NCT03079024; NCT02782442).
The premise of Aim 2B is that the application of effective treatment to improve cognition in early phases of psychosis has a very high likelihood of significantly improving long-term community functioning [40]. We have demonstrated both behavioral gains and improved neural system functioning after neuroscience-informed cognitive training in schizophrenia, in both chronic and early phases of the illness [4,5,41–46]. In young recent-onset individuals (average age of 21 years), our multi-site double-blind randomized controlled trial showed that 40 hours/ 10 weeks of cognitive training delivered at home over a laptop resulted in significant gains in global cognition, verbal memory, and problem solving compared to a computer games control condition [4]. Cognitive gains were significantly correlated with enhanced thalamic volume and thalamo-cortical connectivity, as well as increased white matter integrity ([47], and unpublished data). A meta-analysis of 11 RCT’s in early schizophrenia has indicated the benefit of cognitive remediation approaches [48].
Impaired motivation is also a core feature and very strong predictor of functional outcome in early stages of psychosis [38,49,50]. Some studies have shown positive effects in improving motivation immediately after the intervention (e.g., [51–53]), but treatments that induce enduring improvements in motivated behavior are scarce [7]. Disturbances in motivated behavior reflect a range of factors, including diminished anticipatory pleasure, difficulty learning from rewarding outcomes, reduction in effort expended to obtain rewarding outcomes, and impairment and disconnection between components of social motivation [54–60]. This makes it difficult to determine optimal therapeutic approaches. However, some headway is starting to appear in the literature.
For instance, social cognition impairments appear to play a specific contributing role to dysfunctions in motivated behavior, and are amenable to intervention. We have found that ratings of motivated behavior improve after social cognition training [61], and are significantly greater in subjects who performed cognitive training combined with social cognition training, than in those who completed only cognitive training [6]. We have also demonstrated a significant relationship between 6-month social functioning and training-induced improvements in the neural correlates of a self-other reality monitoring task [45]. These data, along with the literature on reward anticipation and on social engagement in psychosis, led our group to work with young clients in a user-centered design process, to develop a mobile app called Personalized Real-time Intervention for Motivational Enhancement (PRIME [7,62]). The app has been extremely well received by users and recently published behavioral findings are highly promising (see below). Thus, in Aim 2B, we will combine a focused course of cognitive plus social cognitive training (delivered remotely) with PRIME, to address the cognitive dysfunction and impaired motivation identified in Aim 2A.
INNOVATION
We will apply newly emerging computational analytic methods to the longitudinal clinical measurement data acquired for clinician feedback and program evaluation purposes.
We will perform assessments including behavioral measures of cognition and motivated behavior using reliable and scalable digital data acquisition methods. In order to adopt measurement-based care in real-world settings, we must; a) determine which measures provide information that is interpretable and actionable to stake-holders; b) find ways to acquire those measures in reliable, scalable low-cost and low-burden ways.
We will offer personalized feedback sessions (“Findings Visits”) for each individual when they enter care, where we review their assessments and provide brief interpretations of cognition and motivation, emphasizing strengths and discussing areas to work on. We will determine the usefulness in terms of promoting autonomy and participatory decision-making. Follow-up feedback (6 and 12 months) will be offered for those who wish it.
We will employ a scalable neuroscience-informed mobile intervention program to target cognition and motivation, and we will use a cut-off regression discontinuity design to determine its effects.
PRIOR WORK
We have piloted “Findings Visits” on 3 teams, where we provide clients a brief report summarizing their clinical, cognitive, and motivation measures. In a meeting with clients, family members, and primary clinicians, a brief graphical report is provided that includes clinical self-report measures as well as measures from the Brief Assessment of Cognition App [63] and the Behavioral Inhibition Scale/Behavioral Activation Scale [64,65]. The discussion emphasizes each individual’s unique strengths in a sensitive, client-centered manner, with a focus on cognition and motivation. In dialogue with the individual, the team identifies strengths to leverage and potential areas for growth to work on. Qualitatively, the Findings Visits have been unanimously well-received by stakeholders and by the clinical team, who feel empowered by the information.
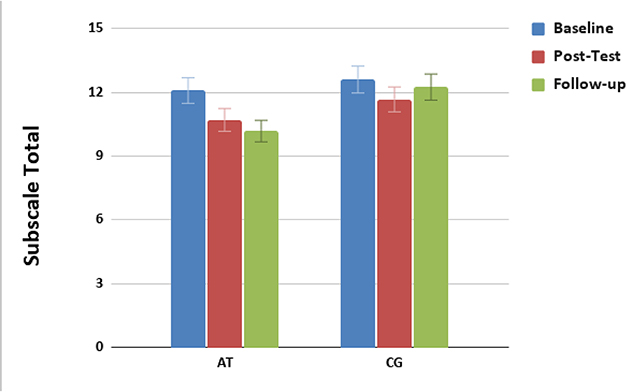
We have implemented mobile cognitive training in people with early psychosis. We have studied the impact of mobile intensive targeted auditory training (versus control condition computer games) immediately after training [4], and at a 6-month follow-up, in a double-blind randomized trial in young adults with recent-onset schizophrenia (under review). 147 randomized participants received laptop computers and instructions to complete 40 hours of training or games over approximately 3 months, along with baseline, post-intervention, and 6-month follow-up assessments of cognition, symptoms, and functioning. A modified intent-to-treat analysis (N = 145) was performed using mixed effects models for repeated measures with planned contrasts based on study completers. Results showed that for Global Cognition, which had improved in the cognitive training group relative to the computer games group at post-training assessments [4], showed durable gains at 6-month follow-up—9 months after study entry. Furthermore, the cognitive training group showed significantly greater improvement in positive symptoms from baseline to 6-month follow-up (Figure 1).
Figure 1.
Baseline (Blue), post training (red), and 6-month follow-up scores (green) in PANSS Positive Symptom ratings in individuals with recent-onset schizophrenia who completed auditory training (AT) versus a computer games control condition (CG).
We performed growth curve modeling on the positive symptom data and found that the cognitive training group showed a significant improvement over time (significant linear slope) whereas the control group did not. These findings suggest that successful cognitive training in the early phases of schizophrenia may result in a significant linear improvement in symptoms over a 9-month period.
We have developed and implemented a mobile app (PRIME) to improve dimensions of motivated behavior. PRIME is a smartphone-based app where participants can interact with their peers and with a coach in what we call the PRIME community. In a feasibility study of PRIME, we conducted two design workshops with 15 key stakeholders (young individuals with a schizophrenia-spectrum disorder, family members, treatment providers, and research experts), and conducted a series of in-depth, 1:1 in-person interviews with six young people with schizophrenia-spectrum disorders [62]. The 1:1 interviews included exercises focused on gaining a better understanding of the values that drive participants to improve their lives; we also asked for detailed feedback on each feature of the app. Users preferred an experience that highlighted principles of Self-Determination Theory, including the desire for more control of their future (autonomy and competence) and an approach that helps them improve existing relationships (relatedness). Ten individuals with recent-onset schizophrenia were then encouraged to use the app daily with a minimum frequency of 1/week over a 12-week period. After 12-weeks, participants used the PRIME app, on average, every other day, were actively engaged with its various features each time they logged in, and retention and satisfaction was high (20/20, 100% retention, high satisfaction ratings).
A second iteration of PRIME was developed with modifications informed by the results of our feasibility study. In an efficacy study of PRIME provided entirely remotely, individuals with early psychosis were recruited from 13 states across the United States and randomized to PRIME (N = 22) or a treatment as usual/waitlist (TAU/WL, N = 21) [7]. Compared to the TAU/WL condition, people in the PRIME condition had significantly greater improvements in self-reported depression, defeatist beliefs, self-efficacy, and trend-level improvement in motivation/pleasure negative symptoms.
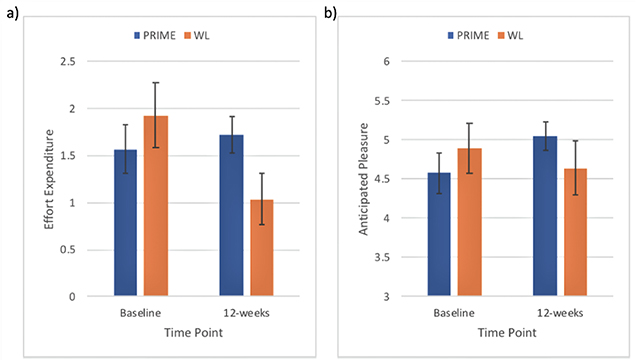
These improvements were maintained 3 months after the end of trial. We also found that people in the PRIME condition had significantly improved performance on a laboratory social motivation task (effort expenditure and anticipated pleasure, Figure 2). Our results suggest that PRIME may serve as an effective mobile intervention for improving aspects of mood and motivation in early psychosis.
Figure 2.
Baseline and post-intervention scores in a laboratory social motivation task after 12 weeks of PRIME app use (blue) vs Wait List (red): (a) Effort expenditure, (b) anticipated pleasure.
METHODS
This project has two main components:
1. Measurement Based Care:
An observational, longitudinal assessment of symptoms, functioning, and cognition delivered using an online platform. Participants engage in a Feedback Visit in which personalized feedback will be delivered through interpretations of the assessment data, emphasizing strengths and discussing areas to work on. Assessments will occur at six month intervals for the duration of their treatment within the coordinated specialty care clinic. We aim to enroll 200 participants per year in this component of the project.
2. Mobile Intervention Targeting Cognition and Motivation:
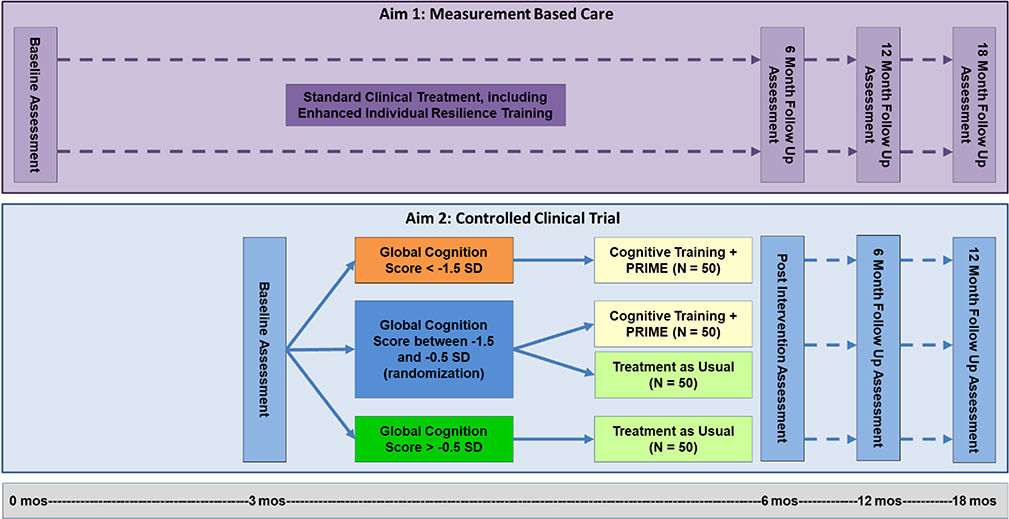
A randomized controlled trial examining 12 weeks of Cognitive Training plus PRIME, with enhanced Individual Resiliency Training (IRT), compared to Treatment as Usual. Participants from the Measurement Based Care component of this project will be invited to participate in this RCT. Participants will first stabilize in their treatment program for approximately 12 weeks, after which they will be invited to participate in the RCT. Participants will be assigned to treatment following the randomization procedure detailed below. Measures will be repeated at Post-Intervention (which corresponds to the 6-month time point in the below timeline), 6-month follow up, and 12-month follow up (Figure 3). Participants may continue to engage in Feedback Visits after each assessment period. We aim to enroll 100 participants per year in this component of the project.
Figure 3.
Study procedure diagram.1 1Estimates of sample sizes after attrition. A total of 400 participants will be recruited for Aim 2.
Randomization to treatment will be conducted by the site study coordinator and will follow a regression discontinuity cut-off based design [66]. Using the participant’s baseline Global Cognition as a cut-off score, they will be assigned to receive the Cognitive Training plus PRIME or Treatment as Usual. Participants with baseline Global Cognition Scores that are lower than −1.5 SD will all be assigned to the mobile intervention. Participants who score above –0.5 SD will be assigned to Treatment as Usual. Those who fall in between these two cut-off scores (between –1.5 and –0.5 SD) will be randomized to receive either Cognitive Training plus PRIME or Treatment as Usual. Participants who are in the Treatment as Usual (TAU) arm will not participate in cognitive training or the PRIME community. They will continue to receive standard care in their coordinated specialty care program, which may include psychotherapy, medication management, psychoeducation, Individual Resiliency Training, work or education support, case management, and peer or family support. Though they will not receive weekly updates from the study team, the Study Coordinator may reach out to them to share important updates about the study during the Intervention Phase.
Since this study uses a regression discontinuity cut-off based design, there is an ethical issue that some participants may not receive the cognitive training intervention. Participants in the treatment as usual group could receive an intervention that is less effective or has unforeseen effects compared to the other group. However, this risk of randomization is not greater than minimal risk, and participants are notified of this risk during the consent process.
Recruitment
Participants will be recruited from one of four clinics around Minnesota that use the NAVIGATE model for treating first episode psychosis. Patients receiving treatment in these clinics will automatically be enrolled in the first aim of the study, which is primarily focused on chart review and measurement based care. Patients will then be approached to participate in the cognitive training portion of this study, which they can opt out of if they choose and must consent to participate. Study coordinators at each clinic will work with clinicians to determine patients who meet eligibility criteria. Study coordinators will communicate with patients and begin the screening process if a patient is interested.
We will attempt to recruit all available participants who meet eligibility criteria, regardless of gender, race, or ethnicity. Our subject sample will reflect the racial and ethnic diversity of our study sites. Based on average demographic information from our clinics, we anticipate that approximately 47% of participants will identify as white/Caucasian, 29% as black/African-American, 5% as Asian, <1% as American Indian/Alaskan, 7% as more than one race, and 12% as Other/Unknown. As more men are affected by psychotic illnesses than women, we expect that 75% of our participants will be male, 22% will be female, and 3% will identify as transgender or other. The average age across the clinics is 23, and participants ages 15–40 will be eligible.
Inclusion and Exclusion Criteria
Participants must have a diagnosis of a first episode psychotic spectrum disorder (onset of symptoms within the past five years or began receiving care in a first episode program within the past five years). They must be receiving treatment in one of four clinics participating in the state of Minnesota that are following the NAVIGATE model. In order to participate in the training, participants must be fluent in written and spoken English, as well as have an estimated IQ of 70 or above, which will be determined by the PENN CNP Matrix Reasoning Test. Patients must have achieved clinical stability before participating, meaning they must have had an outpatient status for at least a month and have reached clinically stable doses of their medication (typically meaning their prescription type or dosage hasn’t changed) for at least a month.
Patients will be excluded from participation if they are unable to provide informed consent, if they are under legal commitment or medical guardianship, if they are diagnosed with a neurological disorder or have clinically significant substance abuse that would impact their participation, if they have participated in a cognitive training program in the past three years, or if they show risk of suicidal behavior, determined by clinician judgement or the Columbia Suicide Severity Rating Scale (C-SSRS).
Emergent Intent for Self or Other Harm
If a research participant expresses suicidal thoughts or plans to harm themselves or others during a research visit, the issue will be addressed during the visit. If the research staff determines that the threat is imminent, The Columbia Suicide Severity Rating Scale(C-SSRS) will be used as an objective suicide screening tool. If a participant endorses some intent to act on suicidal thoughts, they will be referred for further care and evaluation and put on a 30 day hold from the research study. After immediate clinical care has been provided to the participant, research staff will follow-up with the participant’s clinician to ensure that appropriate care is being provided. After the 30 day hold period, the participant will be assessed by a member of the research staff before continuing in the study. The participant will be fully aware of this process and reminded that participating in the study is voluntary.
Clinical Assessments for Measurement Based Care
Early Psychosis Intervention Network (EPINET) designed a Core Assessment Battery (CAB) which includes standardized measures that assess domains of early psychosis. The EPINET CAB domains include symptoms and psychopathology, cognition, recovery, contextual factors and treatment. Data collection from this battery will be included within all four Coordinated Specialty Care clinics in Minnesota.
In addition to the EPINET CAB, all four Coordinated Specialty Care clinics will gather further clinical assessments on diagnosis and symptoms, functioning, quality of life, motivation, illness management and substance use domains. Diagnosis will be assessed with the Mini International Neuropsychiatric Interview-7. Symptoms will be assessed with the, Columbia-Suicide Severity Rating Scale, Calgary Depression Scale for Schizophrenia, and the Symptom Severity Checklist. Functioning will be assessed with the Camberwell Assessment of Need Short Appraisal Schedule, PROMIS-Sleep Disturbance, and International Physical Activity Questionnaire. Quality of life will be assessed using the Abbreviated Quality of Life Scale, Stress Screener for Recent Events, and the Intersectional Discrimination Index. Motivation will be assessed with the Behavioral Inhibition System/Behavioral Activation System. To assess the illness management domain, the Illness Management and Recovery Client and Clinician versions, and Brief Cope assessments will be used. The Alcohol Use Disorder Identification Test and the Drug Abuse Screen Test will be used to assess substance use.
Clinical Assessments for Mobile Intervention Targeting Cognition and Motivation
Participants in Aim 2 will complete the measures above, and the following additional measures of motivation: two self-report assessments—the Motivation and Pleasure Scale-Self Report (MAPS-SR), and the Defeatist Beliefs Subscale from the Dysfunctional Attitudes Scale; and two experimental, computerized tasks of constructs related to motivation. The Effort-Expenditure for Reward Task (EEfRT Task) and the Trust Task.
The EEfRT task presents subjects with a series of repeated trials in which they are able to choose between performing a “hard-task” or an “easy-task” in order to earn varying amounts of monetary reward. In addition to varying reward magnitude, trials are presented with different probability levels for reward receipt, allowing for examination of the extent to which the relationship between motivation levels and effort-based decision-making is modulated by perceived reward magnitude.
During the Trust Task, participants see a name and a dynamic video clip of a social partner displaying an emotional (smile/scowl) or neutral facial expression. Participants then rate their anticipated pleasure for the interaction and decide how much trust (rated between 0–10) to place in the social partner. Points are sent to the social partner, who then returns a percentage of the points. Participants are then presented with the option to expend effort, in the form of key presses, to increase or decrease the likelihood of future interactions with the social partner.
Cognitive and Social Cognitive Training, BrainHQ
We will deliver 20 hours of training, consisting of auditory, executive function, and social cognitive exercises. The rationale for this training schedule is: (1) Significant gains in verbal learning and global cognition were seen in Keefe et al. [67], and in verbal learning and verbal working memory in Popov et al. [68] after 20 h of auditory training; our findings of significant improvements in auditory processing speed associated with generalized gains on cognition after 20 h of auditory training [69]; (2) Social cognition training delivered for 10 hours drives highly robust improvements in our hands [6,70,71] and in the literature [72]; and (3) A lower burden of training reduces attrition and enhances our ability to engage participants in other early intervention services.
Auditory Training Module: This suite of exercises has been extensively studied by us and has been described in detail in Fisher et al. [41]. It is designed to improve the speed and accuracy of auditory information processing while engaging working memory and cognitive control under conditions of close attention and reward. Exercises continuously adjust difficulty level to user performance to maintain an approximately 80% correct performance rate. During the initial stages of training, auditory stimuli are processed to exaggerate the rapid temporal transitions within the stimuli by increasing their amplitude and stretching them in time. The goal of the processing is to increase the effectiveness by which these stimuli engage and drive plastic changes in brain auditory systems that in individuals with psychosis exhibit relatively poor temporal response. This exaggeration is gradually removed so that by the end of training, all auditory stimuli have temporal characteristics representative of real-world rapid speech.
Executive Functioning Module: These training exercises also provide engaging, adaptive training as described above. This module will focus on executive dysfunction, as this domain is a significant, functionally important area of deficit in schizophrenia and has been the target of many previous studies [73,74]. We will train this domain using a suite of engaging, adaptive web-based exercises that target executive function and working memory.
Social Cognition Training Module: This training module consists of exercises designed to ameliorate core deficits in social cognition expressed in schizophrenia and in Autistic Spectrum Disorders. The exercises apply principles of implicit learning to restore the brain’s capacity to process and utilize socially-relevant information, and includes training to improve affect perception (both visual and vocal), social cue perception (in faces, gazes, social situations), theory of mind, self-referential style, and emotion labeling and working memory. This module has been previously studied by us, and drives improvements in social cognition as well as measures of motivated behavior [61].
Personalized Real-Time Motivational Enhancement (PRIME) App
The PRIME smartphone-based app is designed to be used for 12 weeks to enhance motivation in people with early psychosis. Participants work towards self-identified goals with the support of a virtual community of age-matched peers, as well as with motivation coaches [7,62]. Participants discuss their interests and aspirations with each other and with their coach, and the coach sends daily individualized motivational messages. Coaches also provide tailored interventions to enhance motivation, and post daily discussion topics to the PRIME community to encourage interaction between members. Coaches will maintain close communication and feedback on progress with each individual’s clinical team.
Enhanced Individual Resiliency Training (IRT)
An enhanced form of IRT will support clients’ engagement in the Aim 2 research study and help them integrate a focus on cognition into their overall treatment plan. IRT therapists will be trained to evaluate the need for cognitive strategies during the early stages of goal setting and follow-through, how to integrate the cognitive information with treatment and goal planning, and how to collaboratively select and teach cognitive self-management strategies to clients to overcome cognitive challenges to goal attainment (e.g., repeating back information or instructions during a conversation to improve attention and memory, using alarms and schedulers to prompt behavior, steps of planning for a work task or social activity). IRT specialists will collaborate with other members of the team to facilitate follow-through on the client’s use of cognitive self-management strategies. PRIME coaches and IRT therapists will maintain close communication through bi-weekly conference calls.
Power and Statistical Analysis
Aim 1:
At a recruitment rate of 200 individuals per year, we will have baseline data on ~1000 individuals by Year 5. Based on the RAISE-ETP experience, we anticipate an attrition rate of 28% at 6 months (95% CI: 24%–32%) and 36% at 12 months (95% CI: 31%–41%). Thus, we predict that we will have 6- and 12-month data on 720 and 636 individuals respectively, which will provide an adequate sample size to perform the analyses below.
We will apply LCGA to our data set to identify heterogeneous trajectories over time for Quality of Life, symptoms, and work and school participation. We will use M-plus software for latent class growth curve analysis [75] and implement the standard model fitting and selection procedure from the literature [15,76,77]. Specifically, the number of latent classes will be determined through fitting models with progressive number of classes and select the model according to a combination of statistical criteria (which balances goodness of fit and parsimony, such as, Bayesian Information Criterion, BIC; sample-size adjusted BIC; and Akaike Information Criterion, AIC) and interpretability. We will fit progressively more complex growth functions (linear, quadratic) models, where number of measurements over time permits, and select the appropriate complexity. To prevent the model from converging on a local solution, we will run the model on 500 random start values and examine the BIC. Model validity will be tested by comparing the latent class solution in multiple random split-half samples.
To identify the potential variables that are the most predictive of the response pattern a given patient will exhibit, we will employ classifiers including logistic regression [78], support vector machines [79], and random forest [80], feature selection methods including recursive feature elimination [81], regularization [82], and causal feature selection [81]. These methods have been widely applied and shown success in various types of data. We will use repeated nested cross validation to achieve optimal model selection, avoid overfitting, and obtain unbiased performance estimation. Feature selection, missing value treatment, model type and hyper-parameter selection will be conducted in the inner loop of the nested cross-validation, the performance estimation of the selected models will be conducted in the outer loop of the nested cross-validation [22,24,83]. The resulting model will be examined to identify variables that are predictive of the outcome (such as DUP, substance use, treatment intensity, other cognitive, symptom, and functioning variables) and characterize patients that experience improved or worsened outcome. In addition to building predictive models using the raw variables collected, we will reconstruct any latent variables identified from our causal discovery analyses and use them as potential predictors. This will allow us to assess predictive information contained in these latent variables.
To identify causal relationships among baseline variables, we will employ causal discovery methods [84,85]. We will focus on methods that can tolerate the presence of latent confounders, as such confounders are expected to be common in clinical psychiatric data sets. Such methods include GFCI (a recent improvement of the FCI algorithm) [86,87], as well as satisfiability-solver based methods where it is computationally feasible to use them [88]. To identify unmeasured causal factors we will use the Find One Factor Clusters (FOFC) algorithm, due to the algorithm’s performance in simulations compared to other methods and our special expertise with this method [87].
Feature selection will be handled collaboratively between the data scientists and the clinical investigators to ensure that the variables we analyze are both statistically usable and clinically interpretable. Findings will be validated in multiple ways: (1) algorithms will be rerun with varying parameters settings to determine sensitivity to parameter settings; (2) the same algorithms will be run on a secondary data set; (3) resampling methods such as nonparametric bootstrap and jackknife will be used to evaluate output stability; (4) findings will be compared to what is already known in the literature. Latent causal factors discovered with FOFC will additionally be evaluated by determining their usefulness for predicting patient outcomes or personalizing patient treatment.
Aim 2:
During Years ~1–4, 100 individuals will be recruited per year for a total of 400. We anticipate that 20% will withdraw before randomization, for a total of 80 randomizations per year (320 total over 4 years). Based on the RAISE-ETP experience, we anticipate an attrition rate of 28% at the 6-month follow-up (95% CI: 24%–32%) and 36% at the 12 month follow up (95% CI: 31%−41%). Thus, we predict that we will have 6- and 12-month follow up data on 230 and 205 individuals respectively, which will provide an adequate sample size for the analyses described below.
We estimated power via simulation for several outcomes based on the design proposed in Figure 3. We assumed that Baseline Global Cognition scores were generated from the distribution observed in RAISE, and simulated change scores for outcomes from a normal distribution with means depending on the hypothesized intervention effect and standard deviations estimated from the literature. For the outcome of Global Cognition, our sample size of n = 200 (100 per group, with half of these randomized) has approximately 85% power to detect an effect of 0.35 SD units on change in Global Cognition, which is comparable to the effect seen in our study of auditory training in recent-onset schizophrenia [4]. It also provides 80% power to detect a 0.7 unit effect on change in motivation, comparable to the effect observed in our efficacy study of PRIME [7], and an effect of 5 units on change in Quality of Life Scale Total Score [89], which is a clinically meaningful level of improvement [90].
The primary analysis will compare changes from pre to post intervention in cognition, motivation, and functioning. After assessing for normality (and log-transforming if necessary), we will fit linear regression models to pre-post differences in outcomes. Since intervention status is only partially randomized, the model will contain a main effect for intervention status and also adjust for baseline cognition score, symptom severity, duration of untreated psychosis, QLS, age, sex, race, and clinic. Two-sided hypothesis tests will be performed on the intervention main effect using a significance level of 0.05. One key sensitivity analysis will involve estimating the intervention effect from the subset of n = 100 randomized participants only. If the estimated intervention effect differs substantially between the randomized subset and the overall study population, primary and secondary analyses will be reported separately for these groups.
In secondary analyses, we will fit linear mixed effects (i.e., growth curve) models to characterize longitudinal changes in outcomes immediately post-intervention, 6 months post-intervention, and 12 months post-intervention. We will also fit models with interaction terms between intervention and various baseline characteristics to ascertain whether intervention effectiveness differs within particular subgroups.
Finally, we will test if dropout is at random given the observed data, and compare participants who complete the study to participants who drop out on a number of variables including demographics, symptom severity, functioning, motivation and quality of life, to determine if there are specific reasons for dropout. We will also examine potential different reasons for dropout for each treatment group.
CHALLENGES AND LIMITATIONS
It is important to note that most FEP teams currently thriving in the US are located in large population areas, due to low incidence rates of schizophrenia. These low incidence rates lead to extensive challenges for part-time teams that work outside of metropolitan areas. One such site in our hub is outside of the metropolitan area. As a rural site, the team is not expected to enroll participants at the same rate as a large metropolitan team. Although small, it is an important site included in our sample as their NAVIGATE program recruits from a large rural region in Minnesota, increasing our ability to study how to deliver optimal services to large rural areas.
We anticipate a fairly substantial amount of attrition in this study population. Our primary longitudinal analysis will be conducted on subjects who complete the 6 month post-intervention follow-up assessments (which is the 12-month assessment time point after first entering treatment). Secondarily, we will also examine longer term changes by growth curve analysis using baseline 6-month, 12-month and 18-month assessments. We will also perform sensitivity analyses to determine the effect of excluding patients with missing outcome assessments.
We hypothesize that individual- and clinic-specific factors will contribute to differential drop-out in the two arms. We will build models for early dropout to identify these factors, and use the results to carry out inverse probability of treatment weighted (IPW) analyses to estimate the causal effects of the intervention. These analyses can guide a more comprehensive interpretation of the effectiveness of the treatment and improve adherence in future clinical deployment.
SUMMARY AND EXPECTED CONTRIBUTIONS TO THE FIELD
Functional recovery lags behind symptom recovery in early intervention programs, is sometimes difficult for individuals to attain, and is closely aligned with cognitive and motivational deficits [91]. The use of measurement-based care may lead to increased satisfaction with care, more refined, personalized courses of treatment, and the identification of specific treatment targets. The results from Aim 1 of this project will provide data-driven knowledge on factors that contribute to treatment response trajectories in early psychosis. This knowledge will be combined with insights from AIM 2 to deepen our understanding of methods to optimize coordinated specialty care, using a well-defined scalable mobile program to target cognitive dysfunction and motivation impairment. These core features of psychotic illness directly impact an individual’s ability to recover from the illness and re-integrate into society by pursuing education and gainful employment. If the cognitive training and motivational enhancement intervention is effective, it will provide a non-pharmacological treatment approach for young people that is safe and easy to apply, and that may improve long-term outcome, reducing burden on individuals, their families, and their communities.
Acknowledgments
FUNDING
This project is funded by the National Institutes of Health R01 MH120589-01.
Footnotes
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
REFERENCES
- 1.Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, et al. Global Epidemiology and Burden of Schizophrenia: Findings From the Global Burden of Disease Study 2016. Schizophr Bull. 2018;44(6):1195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jääskeläinen E, Juola P, Hirvonen N, McGrath JJ, Saha S, Isohanni M, et al. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull. 2013;39(6):1296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kane JM, Robinson DG, Schooler NR, Mueser KT, Penn DL, Rosenheck RA, et al. Comprehensive Versus Usual Community Care for First-Episode Psychosis: 2-Year Outcomes From the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016. April 1;173(4):362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher M, Loewy R, Carter C, Lee A, Ragland JD, Niendam T, et al. Neuroplasticity-based auditory training via laptop computer improves cognition in young individuals with recent onset schizophrenia. Schizophr Bull. 2015;41(1):250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loewy R, Fisher M, Carter C, Ragland JD, Niendam T, Stuart B, et al. Durable Cognitive Gains and Symptom Improvement are Observed in Individuals with Recent-Onset Schizophrenia Six Months after a Randomized Trial of Mobile Auditory Training. 2020. Unpublished work. [DOI] [PMC free article] [PubMed]
- 6.Fisher M, Nahum M, Howard E, Rowlands A, Brandrett B, Kermott A, et al. Supplementing intensive targeted computerized cognitive training with social cognitive exercises for people with schizophrenia: An interim report. Psychiatr Rehabil J. 2017;40(1):21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlosser DA, Campellone TR, Truong B, Etter K, Vergani S, Komaiko K, et al. Efficacy of PRIME, a Mobile App Intervention Designed to Improve Motivation in Young People With Schizophrenia. Schizophr Bull. 2018;44(5):1010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas S, Button S, Casey SE. Implementing for Sustainability: Promoting Use of a Measurement Feedback System for Innovation and Quality Improvement. Adm Policy Ment Health. 2016;43(3):286–91. [DOI] [PubMed] [Google Scholar]
- 9.Fortney JC, Unützer J, Wrenn G, Pyne JM, Smith GR, Schoenbaum M, et al. A Tipping Point for Measurement-Based Care. Focus. 2018;16(3):341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo T, Xiang Y-T, Xiao L, Hu C-Q, Chiu HFK, Ungvari GS, et al. Measurement-Based Care Versus Standard Care for Major Depression: A Randomized Controlled Trial With Blind Raters. Am J Psychiatry. 2015;172(10):1004–13. [DOI] [PubMed] [Google Scholar]
- 11.Harding KJK, Rush AJ, Arbuckle M, Trivedi MH, Pincus HA. Measurement-based care in psychiatric practice: a policy framework for implementation. J Clin Psychiatry. 2011;72(8):1136–43. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhry B, Wang J, Wu S, Maglione M, Mojica W, Roth E, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006. May 16;144(10):742–52. Available from: 10.7326/0003-4819-144-10-200605160-00125 [DOI] [PubMed] [Google Scholar]
- 13.Scott K, Lewis CC. Using Measurement-Based Care to Enhance Any Treatment. Cogn Behav Pract. 2015;22(1):49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bickman L, Lyon AR, Wolpert M. Achieving Precision Mental Health through Effective Assessment, Monitoring, and Feedback Processes. Admin Policy Mental Health Ment Health Res. 2016;43(3):271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curran PJ, Muthén BO. The application of latent curve analysis to testing developmental theories in intervention research. Am J Community Psychol. 1999;27(4):567–95. [DOI] [PubMed] [Google Scholar]
- 16.Meredith W, Tisak J. Latent curve analysis. Psychometrika. 1990;55(1):107–22. [Google Scholar]
- 17.Jung T, Wickrama KAS. An Introduction to Latent Class Growth Analysis and Growth Mixture Modeling. Soc Personal Psychol Compass. 2008;2(1):302–17. [Google Scholar]
- 18.Muthen B, Muthen LK. Integrating Person-Centered and Variable-Centered Analyses: Growth Mixture Modeling With Latent Trajectory Classes. Alcohol Clin Exp Res. 2000;24:882–91. [PubMed] [Google Scholar]
- 19.Galatzer-Levy IR, Ma S, Statnikov A, Yehuda R, Shalev AY. Utilization of machine learning for prediction of post-traumatic stress: a re-examination of cortisol in the prediction and pathways to non-remitting PTSD. Transl Psychiatry. 2017;7(3):e0. doi: 10.1038/tp.2017.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llabre MM, Spitzer S, Siegel S, Saab PG, Schneiderman N. Applying latent growth curve modeling to the investigation of individual differences in cardiovascular recovery from stress. Psychosom Med. 2004;66(1):29–41. [DOI] [PubMed] [Google Scholar]
- 21.Beauchaine TP, Webster-Stratton C, Reid MJ. Mediators, moderators, and predictors of 1-year outcomes among children treated for early-onset conduct problems: a latent growth curve analysis. J Consult Clin Psychol. 2005;73(3):371–88. [DOI] [PubMed] [Google Scholar]
- 22.Friedman J, Hastie T, Tibshirani R. The elements of statistical learning. New York (US): Springer; 2001. [Google Scholar]
- 23.Duda RO, Hart PE, Stork DG. Pattern Classification. Hoboken (US): John Wiley & Sons; 2012. p. 688. [Google Scholar]
- 24.Statnikov A, Aliferis CF, Hardin DP, Guyon I. A Gentle Introduction to Support Vector Machines in Biomedicine: Volume 1: Theory and Methods. Hackensack (US): World Scientific Publishing Company; 2011. p. 200. [Google Scholar]
- 25.Ma S, Galatzer-Levy IR, Wang X, Fenyö D, Shalev AY. A First Step towards a Clinical Decision Support System for Post-traumatic Stress Disorders. AMIA Annu Symp Proc. 2016;2016:837–43. [PMC free article] [PubMed] [Google Scholar]
- 26.Iwabuchi SJ, Liddle PF, Palaniyappan L. Clinical utility of machine-learning approaches in schizophrenia: improving diagnostic confidence for translational neuroimaging. Front Psychiatry. 2013;4:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saxe GN, Ma S, Ren J, Aliferis C. Machine learning methods to predict child posttraumatic stress: a proof of concept study. BMC Psychiatry. 2017;17(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koutsouleris N, Kahn RS, Chekroud AM, Leucht S, Falkai P, Wobrock T, et al. Multisite prediction of 4-week and 52-week treatment outcomes in patients with first-episode psychosis: a machine learning approach. Lancet Psychiatry. 2016;3(10):935–46. [DOI] [PubMed] [Google Scholar]
- 29.Milev P, Ho B-C, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495–506. [DOI] [PubMed] [Google Scholar]
- 30.Bowie CR, Harvey PD. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr Dis Treat. 2006;2(4):531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green MF. Impact of cognitive and social cognitive impairment on functional outcomes in patients with schizophrenia. J Clin Psychiatry. 2016;77(Suppl 2):8–11. [DOI] [PubMed] [Google Scholar]
- 32.Bosanac P, Patton GC, Castle DJ. Early intervention in psychotic disorders: faith before facts? Psychol Med. 2010;40(3):353–8. [DOI] [PubMed] [Google Scholar]
- 33.Hegelstad WTV, Larsen TK, Auestad B, Evensen J, Haahr U, Joa I, et al. Long-term follow-up of the TIPS early detection in psychosis study: effects on 10-year outcome. Am J Psychiatry. 2012;169(4):374–80. [DOI] [PubMed] [Google Scholar]
- 34.Najas-Garcia A, Gómez-Benito J, Huedo-Medina TB. The Relationship of Motivation and Neurocognition with Functionality in Schizophrenia: A Meta-analytic Review. Community Ment Health J. 2018;54(7):1019–49. [DOI] [PubMed] [Google Scholar]
- 35.Barch DM. The relationships among cognition, motivation, and emotion in schizophrenia: how much and how little we know. Schizophr Bull. 2005;31(4):875–81. [DOI] [PubMed] [Google Scholar]
- 36.Medalia A, Saperstein A. The role of motivation for treatment success. Schizophr Bull. 2011;37(Suppl 2):S122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fervaha G, Foussias G, Agid O, Remington G. Motivational and neurocognitive deficits are central to the prediction of longitudinal functional outcome in schizophrenia. Acta Psychiatr Scand. 2014;130(4):290–9. [DOI] [PubMed] [Google Scholar]
- 38.Fervaha G, Foussias G, Agid O, Remington G. Motivational deficits in early schizophrenia: prevalent, persistent, and key determinants of functional outcome. Schizophr Res. 2015;166(1–3):9–16. [DOI] [PubMed] [Google Scholar]
- 39.Takeda K, Sumiyoshi T, Matsumoto M, Murayama K, Ikezawa S, Matsumoto K, et al. Neural Correlates for Intrinsic Motivational Deficits of Schizophrenia; Implications for Therapeutics of Cognitive Impairment. Front Psychiatry. 2018;9:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nuechterlein KH, Subotnik KL, Green MF, Ventura J, Asarnow RF, Gitlin MJ, et al. Neurocognitive predictors of work outcome in recent-onset schizophrenia. Schizophr Bull. 2011;37(Suppl 2):S33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher M, Holland C, Merzenich MM, Vinogradov S. Using Neuroplasticity-Based Auditory Training to Improve Verbal Memory in Schizophrenia. Am J Psychiatry. 2009;166(7):805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher M, Holland C, Subramaniam K, Vinogradov S. Neuroplasticity-based cognitive training in schizophrenia: an interim report on the effects 6 months later. Schizophr Bull. 2010;36(4):869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loewy R, Fisher M, Schlosser DA, Biagianti B, Stuart B, Mathalon DH, et al. Intensive Auditory Cognitive Training Improves Verbal Memory in Adolescents and Young Adults at Clinical High Risk for Psychosis. Schizophr Bull. 2016;42(Suppl 1):S118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adcock RA, Dale C, Fisher M, Aldebot S, Genevsky A, Simpson GV, et al. When Top-Down Meets Bottom-Up: Auditory Training Enhances Verbal Memory in Schizophrenia. Vol. 35, Schizophr Bull. 2009;35:1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73(4):842–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subramaniam K, Luks TL, Garrett C, Chung C, Fisher M, Nagarajan S, et al. Intensive cognitive training in schizophrenia enhances working memory and associated prefrontal cortical efficiency in a manner that drives long-term functional gains. NeuroImage. 2014;99:281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramsay IS, Fryer S, Boos A, Roach BJ, Fisher M, Loewy R, et al. Response to Targeted Cognitive Training Correlates with Change in Thalamic Volume in a Randomized Trial for Early Schizophrenia. Neuropsychopharmacology. 2018;43(3):590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Revell ER, Neill JC, Harte M, Khan Z, Drake RJ. A systematic review and meta-analysis of cognitive remediation in early schizophrenia. Schizophr Res. 2015;168(1–2):213–22. [DOI] [PubMed] [Google Scholar]
- 49.Chang WC, Kwong VWY, Or Chi Fai P, Lau ESK, Chan GHK, Jim OTT, et al. Motivational impairment predicts functional remission in first-episode psychosis: 3-Year follow-up of the randomized controlled trial on extended early intervention. Aust N Z J Psychiatry. 2018;52(12):1194–201. [DOI] [PubMed] [Google Scholar]
- 50.Chang WC, Hui CLM, Chan SKW, Lee EHM, Chen EYH. Impact of avolition and cognitive impairment on functional outcome in first-episode schizophrenia-spectrum disorder: a prospective one-year follow-up study. Schizophr Res. 2016;170(2–3):318–21. [DOI] [PubMed] [Google Scholar]
- 51.Lee H-S, Jang S-K, Lee G-Y, Park S-C, Medalia A, Choi K-H. Informationally administered reward enhances intrinsic motivation in schizophrenia. Psychiatry Res. 2017;256:290–7. [DOI] [PubMed] [Google Scholar]
- 52.Choi J, Medalia A. Intrinsic motivation and learning in a schizophrenia spectrum sample. Schizophr Res. 2010;118(1–3):12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fiszdon JM, Kurtz MM, Choi J, Bell MD, Martino S. Motivational Interviewing to Increase Cognitive Rehabilitation Adherence in Schizophrenia. Schizophr Bull. 2016;42(2):327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reddy LF, Horan WP, Green MF. Motivational Deficits and Negative Symptoms in Schizophrenia: Concepts and Assessments. Curr Top Behav Neurosci. 2016;27:357–73. [DOI] [PubMed] [Google Scholar]
- 55.Kring AM, Barch DM. The motivation and pleasure dimension of negative symptoms: neural substrates and behavioral outputs. Eur Neuropsychopharmacol. 2014;24(5):725–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull. 2014;40(Suppl 2):S107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008. September;34(5):835–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCarthy JM, Treadway MT, Bennett ME, Blanchard JJ. Inefficient effort allocation and negative symptoms in individuals with schizophrenia. Schizophr Res. 2016;170(2–3):278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Treadway MT, Peterman JS, Zald DH, Park S. Impaired effort allocation in patients with schizophrenia. Schizophr Res. 2015;161(2–3):382–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campellone TR, Truong B, Gard D, Schlosser DA. Social motivation in people with recent-onset schizophrenia spectrum disorders. J Psychiatr Res. 2018;99:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nahum M, Fisher M, Loewy R, Poelke G, Ventura J, Nuechterlein KH, et al. A novel, online social cognitive training program for young adults with schizophrenia: A pilot study. Schizophr Res Cogn. 2014;1(1):e11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schlosser D, Campellone T, Kim D, Truong B, Vergani S, Ward C, et al. Feasibility of PRIME: A Cognitive Neuroscience-Informed Mobile App Intervention to Enhance Motivated Behavior and Improve Quality of Life in Recent Onset Schizophrenia. JMIR Re Protoc. 2016;5:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Atkins AS, Tseng T, Vaughan A, Twamley EW, Harvey P, Patterson T, et al. Validation of the tablet-administered Brief Assessment of Cognition (BAC App). Vol. 181, Schizophr Res. 2017;181:100–6. [DOI] [PubMed] [Google Scholar]
- 64.Schlosser DA, Fisher M, Gard D, Fulford D, Loewy RL, Vinogradov S. Motivational deficits in individuals at-risk for psychosis and across the course of schizophrenia. Schizophr Res. 2014;158(1–3):52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J Pers Soc Psychol. 1994;67(2):319–33. [Google Scholar]
- 66.Cappelleri JC, Trochim WM. Ethical and scientific features of cutoff-based designs of clinical trials: a simulation study. Med Decis Making. 1995;15(4):387–94. [DOI] [PubMed] [Google Scholar]
- 67.Keefe RSE, Vinogradov S, Medalia A, Buckley PF, Caroff SN, D’Souza DC, et al. Feasibility and Pilot Efficacy Results From the Multisite Cognitive Remediation in the Schizophrenia Trials Network (CRSTN) Randomized Controlled Trial. Vol. 73, J Clin Psychiatry. 2012;73:1016–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Popov T, Jordanov T, Rockstroh B, Elbert T, Merzenich MM, Miller GA. Specific cognitive training normalizes auditory sensory gating in schizophrenia: a randomized trial. Biol Psychiatry. 2011;69(5):465–71. [DOI] [PubMed] [Google Scholar]
- 69.Biagianti B, Fisher M, Neilands TB, Loewy R, Vinogradov S. Engagement with the auditory processing system during targeted auditory cognitive training mediates changes in cognitive outcomes in individuals with schizophrenia. Neuropsychology. 2016;30(8):998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sacks S, Fisher M, Garrett C, Alexander P, Holland C, Rose D, et al. Combining Computerized Social Cognitive Training with Neuroplasticity-Based Auditory Training in Schizophrenia. Clin Schizophr Related Psych. 2013;7:78–86A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hooker CI, Bruce L, Fisher M, Verosky SC, Miyakawa A, Vinogradov S. Neural activity during emotion recognition after combined cognitive plus social cognitive training in schizophrenia. Vol. 139, Schizophre Res. 2012;139:53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grynszpan O, Perbal S, Pelissolo A, Fossati P, Jouvent R, Dubal S, et al. Efficacy and specificity of computer-assisted cognitive remediation in schizophrenia: a meta-analytical study. Psychol Med. 2011;41(1):163–73. [DOI] [PubMed] [Google Scholar]
- 73.Medalia A, Revheim N, Casey M. Remediation of problem-solving skills in schizophrenia: evidence of a persistent effect. Schizophr Res. 2002;57(2–3):165–71. [DOI] [PubMed] [Google Scholar]
- 74.Wykes T, Newton E, Landau S, Rice C, Thompson N, Frangou S. Cognitive remediation therapy (CRT) for young early onset patients with schizophrenia: an exploratory randomized controlled trial. Schizophr Res. 2007;94(1–3):221–30. [DOI] [PubMed] [Google Scholar]
- 75.Muthén LK, Muthén BO. Mplus: Statistical Analysis with Latent Variables—User’s Guide (2012). Available from: http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.310.2841. Accessed 2020 Oct 14.
- 76.Kline RB. Principles and practice of structural equation modeling. New York (US): Guilford publications; 2015. [Google Scholar]
- 77.Hooper D, Coughlan J, Mullen M. Structural equation modelling: Guidelines for determining model fit. Electron J Bus Res Methods. 2008;6(1):53–60. [Google Scholar]
- 78.Cox DR. The Regression Analysis of Binary Sequences. J R Stat Soc Series B Stat Methodol. 1958;20(2):215–42. [Google Scholar]
- 79.Boser BE, Guyon IM, Vapnik VN. A Training Algorithm for Optimal Margin Classifiers. In: Proceedings of the Fifth Annual Workshop on Computational Learning Theory New York (NY, USA): ACM; 1992. p. 144–52. [Google Scholar]
- 80.Breiman L Random Forests. Mach Learn. 2001;45(1):5–32. [Google Scholar]
- 81.Guyon I, Aliferis C, Elisseeff A. Causal feature selection. methods of feature selection. 2007;63–82. [Google Scholar]
- 82.Tibshirani R Regression Shrinkage and Selection via the Lasso. J R Stat Soc Series B Stat Methodol. 1996;58(1):267–88. [Google Scholar]
- 83.Murphy KP. Machine Learning: A Probabilistic Perspective. Cambridge (US): MIT Press; 2012. p. 1104. [Google Scholar]
- 84.Pearl J Causality. Cambridge (UK): Cambridge University Press; 2009. p. 486. [Google Scholar]
- 85.Spirtes P, Glymour CN, Scheines R. Causation, Prediction, and Search. 2nd ed. Cambridge (US): MIT Press; 2000. p. 543. [Google Scholar]
- 86.Spirtes P, Meek C, Richardson T. Causal inference in the presence of latent variables and selection bias. In: Proceedings of the Eleventh conference on Uncertainty in artificial intelligence San Francisco (CA, US): Morgan Kaufmann Publishers Inc.; 1995. p. 499–506. [Google Scholar]
- 87.Ogarrio JM, Spirtes P, Ramsey J. A Hybrid Causal Search Algorithm for Latent Variable Models. JMLR Workshop Conf Proc 2016;52:368–79. [PMC free article] [PubMed] [Google Scholar]
- 88.Hyttinen A, Eberhardt F, Järvisalo M. Constraint-based causal discovery: conflict resolution with answer set programming. In: Proceedings of the Thirtieth Conference on Uncertainty in Artificial Intelligence Arlington (US): AUAI Press; 2014. p. 340–9. [Google Scholar]
- 89.Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388–98. [DOI] [PubMed] [Google Scholar]
- 90.Cramer J, Rosenheck R, Xu W, Henderson W, Thomas J, Charney D, et al. Detecting improvement in quality of life and symptomatology in schizophrenia. Schizophr Bull. 2001;27(2):227–34. [DOI] [PubMed] [Google Scholar]
- 91.Robinson DG, Woerner MG, McMeniman M, Mendelowitz A, Bilder RM. Symptomatic and functional recovery from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 2004;161(3):473–9. [DOI] [PubMed] [Google Scholar]