Figure 5. Reconstructing Evolution of a Broadly Binding High-Affinity Anti-influenza Antibody Clone.
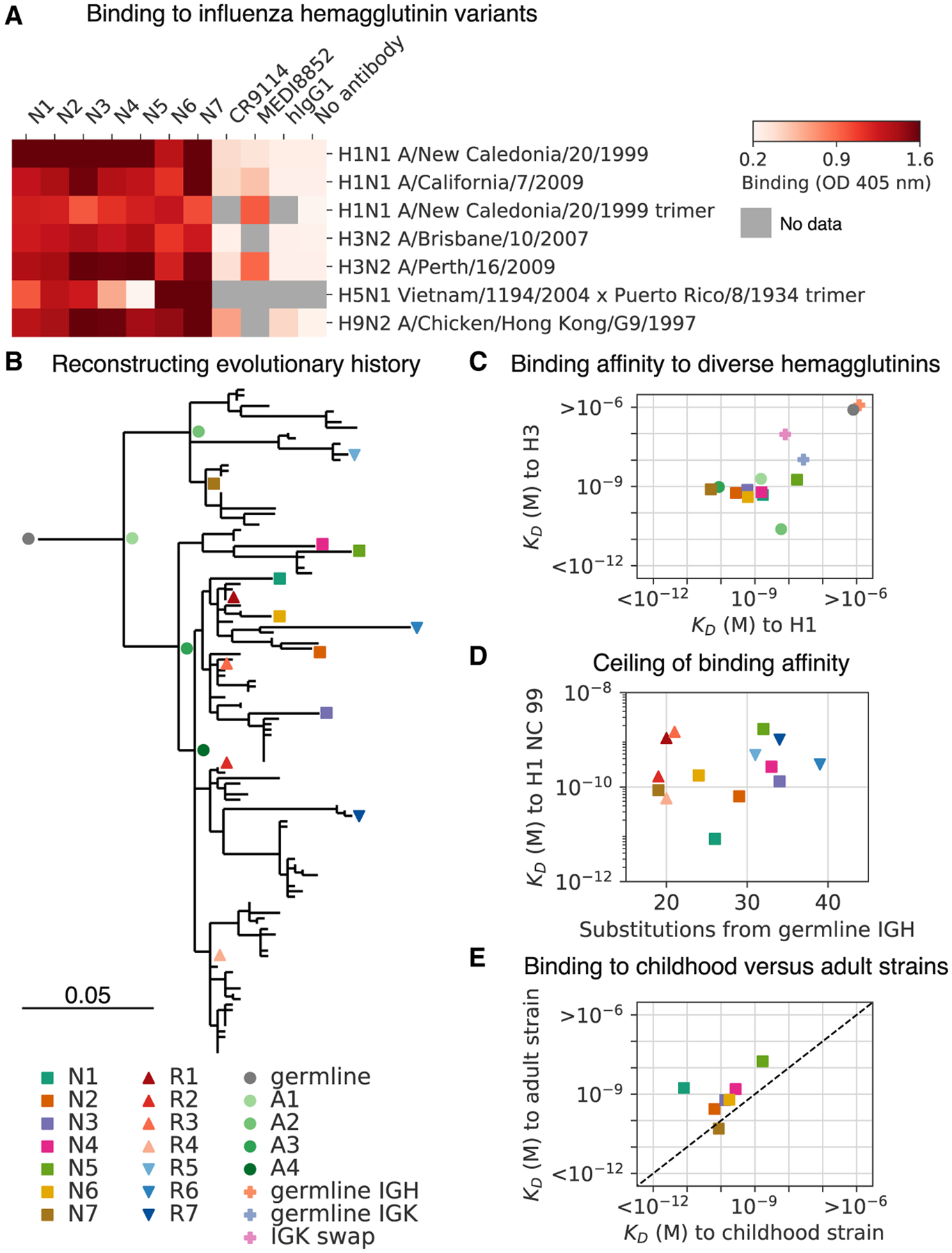
(A) Binding of antibodies from the L3 clone to a panel of influenza hemagglutinin (HA) variants was measured using ELISA. OD, optical density; hIgG1, human IgG1.
(B) Evolutionary history of L3 depicted as a maximum-likelihood phylogeny based on heavy-chain sequence. Markers indicate antibodies detected by single-cell sequencing (N1–N7) or repertoire sequencing (R1–R7), or reconstructed ancestral sequences (germline and A1–A4).
(C) Dissociation constants (KDs) of binding between L3 antibody variants and H1 (A/California/7/2009) and H3 (A/Perth/16/2009) hemagglutinin variants, as determined by biolayer interferometry. L3 antibodies include extant sequences (N1–N7), reconstructed ancestral sequences (germline and A1–A4), and engineered variants having the L3N6 sequences, but with heavy chain reverted to the inferred germline sequence (germline immunoglobulin heavy chain [IGH]), light chain reverted to the inferred germline sequence (germline immunoglobulin kappa chain [IGK]), or a light chain sequence substituted from a different clone (IGK swap). Jitter was added to germline and germline IGH to improve visualization of the data points.
(D) Dissociation constants of binding between L3 antibodies compared with extent of somatic hypermutation.
(E) Dissociation constants of binding between L3 antibodies and H1 variants from childhood (A/New Caledonia/20/1999) and adulthood (A/California/7/2009). Dashed line indicates equal KD for binding to both variants. Uncertainty of fitted parameters was smaller than the size of the markers used for plotting; therefore, error bars are not shown.
