Abstract
STUDY QUESTION
Do mitochondria-targeted therapies reverse ageing- and oxidative stress-induced spindle defects in oocytes from mice and humans?
SUMMARY ANSWER
Exposure to MitoQ or BGP-15 during IVM protected against spindle and chromosomal defects in mouse oocytes exposed to oxidative stress or derived from reproductively aged mice whilst MitoQ promoted nuclear maturation and protected against chromosomal misalignments in human oocytes.
WHAT IS KNOWN ALREADY
Spindle and chromosomal abnormalities in oocytes are more prevalent with maternal aging, increasing the risk of aneuploidy, miscarriage and genetic disorders such as Down’s syndrome. The origin of compromised oocyte function may be founded in mitochondrial dysfunction and increased reactive oxygen species (ROS).
STUDY DESIGN, SIZE, DURATION
Oocytes from young and old mice were treated with MitoQ and/or BGP-15 during IVM. To directly induce mitochondrial dysfunction, oocytes were treated with H2O2, and then treated the MitoQ and/or BGP-15. Immature human oocytes were cultured with or without MitoQ. Each experiment was repeated at least three times, and data were analyzed by unpaired-sample t-test or chi-square test.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Immature germinal vesicle (GV) stage oocytes from 1-, 12- and 18-month-old mice were obtained from preovulatory ovarian follicles. Oocytes were treated with MitoQ and/or BGP-15 during IVM. GV-stage human oocytes were cultured with or without MitoQ. Mitochondrial membrane potential and mitochondrial ROS were measured by live-cell imaging. Meiotic spindle and chromosome alignments were visualized by immunofluorescent labeling of fixed oocytes and the 3-dimensional images were analyzed by Imaris.
MAIN RESULTS AND THE ROLE OF CHANCE
MitoQ or BGP-15 during IVM protects against spindle and chromosomal defects in oocytes exposed to oxidative stress and in oocytes from aged mice (P < 0.001). In human oocytes, the presence of MitoQ during IVM promoted nuclear maturation and had a similar positive effect in protecting against chromosomal misalignments (P < 0.001).
LIMITATIONS, REASONS FOR CAUTION
Our study identifies two excellent candidates that may help to improve fertility in older women. However, these potential therapies must be tested for efficacy in clinical IVM systems, and undergo thorough examination of resultant offspring in preclinical models before utilization.
WIDER IMPLICATIONS OF THE FINDINGS
Our results using in-vitro systems for oocyte maturation in both mouse and human provide proof of principle that mitochondrially targeted molecules such as MitoQ and BGP-15 may represent a novel therapeutic approach against maternal aging-related spindle and chromosomal abnormalities.
STUDY FUNDING/COMPETING INTEREST(S)
The project was financially supported by the National Health and Medical Research Council and Australian Research Council, Australia. U.A.-Z. was supported by the Iraqi Higher Education and Scientific Research Ministry PhD scholarship and O.C. was supported by TUBITAK-1059B191601275. M.P.M. consults for MitoQ Inc. and holds patents in mitochondria-targeted therapies. R.L.R. is an inventor on patents relating to the use of BGP-15 to improve gamete quality.
TRIAL REGISTRATION NUMBER
N/A
Keywords: aging, oocyte, spindle, chromosome, mitochondria-targeted therapeutics
Introduction
It is well established that maternal aging is associated with a decrease in oocyte quality leading to infertility and an increased risk of miscarriage and genetic anomalies such as Down’s syndrome. Decreased fertility in women occurs gradually from the age of 30 but undergoes a precipitous decline between the ages of 35 and 40 (Toner and Flood, 1993; Baird et al., 2005). Thus, the societal trend to delay childbearing (Sauer, 2015) is leading to increasing numbers of couples seeking ART due to age-related infertility. The age-related decrease in female fertility is due to a steep decline in both ovarian reserve and oocyte quality. This process reflects the established dogma that all oocytes are formed during fetal life, are non-renewing and remain arrested in a non-growing state for what may be decades before being recruited to grow and mature. Oocyte quality is understood to be the primary limiting factor for female fertility with aging because donor oocytes from young women result in IVF success rates consistent with those of the young donor rather than the older recipient (Wang et al., 2012). Thus, deteriorating oocyte quality is the major cause of fertility decline with advanced maternal age.
Production of a developmentally competent egg requires resumption and successful completion of two successive meiotic divisions. The decrease in fertility and increase in miscarriage rate associated with maternal age may be caused by increase in the rate of chromosomal errors (Hassold et al., 2007; Nagaoka et al., 2012). In fact, by the age of 40, the rate of aneuploidy in oocytes has been reported to be as high as 60% (Angell, 1994; Pellestor et al., 2003). Thus, there is a pressing need to understand the molecular mechanisms behind age-related spindle and chromosomal abnormalities in oocytes and to develop therapeutic measures tackling this problem.
Multiple molecular defects contribute to the deterioration of oocyte quality and the occurrence of higher rates of aneuploidy with maternal aging (reviewed in: Nagaoka et al., 2012; Jones and Lane, 2013; Schatten and Sun, 2015; Touati and Wassmann, 2016; Greaney et al., 2018; Kitajima, 2018). The major candidate mechanisms for increased aneuploidy in maternal aging include progressive loss of cohesion (Chiang et al., 2010; Lister et al., 2010) and a compromised spindle assembly checkpoint to monitor chromosome attachment errors (Lane and Jones, 2014; Marangos et al., 2015; Nabti et al., 2017). In addition, age-related defects in microtubule dynamics are also apparent in mouse eggs and may contribute to age-associated chromosome segregation errors (Nakagawa and FitzHarris, 2017).
In addition to these defects in the meiotic machinery, mitochondrial dysfunction is considered to be a major factor contributing to aging processes. Mitochondrial electron transport chain coupled oxidative phosphorylation (OXPHOS) is responsible for most of the ATP generated in oocytes (Dalton et al., 2014). However, one by-product of OXPHOS is the generation of potentially damaging reactive oxygen species (ROS). Mitochondrial dysfunction that causes a decrease in ATP and/or an increase in ROS is sufficient to disrupt meiotic spindles (Zhang et al., 2006; Zeng et al., 2007). General antioxidants such as Coenzyme Q10 (CoQ10) (Ben-Meir et al., 2015) and N-acetyl-l-cysteine (Liu et al., 2012) as well as other small molecule like resveratrol (Liu et al., 2013) are thought to improve mitochondrial function. Most recently nicotinamide mononucleotide (Bertoldo et al., 2020) has been shown to improve reproductive outcome in mouse models of ovarian aging.
Mitochondria-targeted therapeutics are now being studied in a number of aging models because they have shown greater potential in scavenging ROS generated by mitochondria than the untargeted general antioxidants. Their effectiveness is thought to be related to the ability of these molecules to readily cross the mitochondrial phospholipid bilayer and accumulate within the mitochondrial matrix thus decreasing oxidative damage at the site of ROS production (Oyewole and Birch-Machin, 2015; Murphy, 2016; Young and Franklin, 2019). MitoQ (mitoquinone mesylate: (10-(4,5-Dimethoxy-2-methyl-3,6-dioxo-1,4-cyclohexadien-1-yl)decyl)(triphenyl)phosphonium methanesulfonate) is a ubiquinone moiety linked to a lipophilic triphenylphosphonium cation by a 10-carbon alkyl chain that preferentially accumulates in mitochondria (Murphy and Smith, 2007). A second molecule BGP-15 ((O-(3-piperidino-2-hydroxy-1-propyl)-nicotinic amidoxime)) is a hydroximic acid niacin derivative characterized as an insulin sensitizer. It has been shown to preferentially accumulate in mitochondria (Sumegi et al., 2017) and although its precise mode of action remains unclear it has been demonstrated to decrease mitochondrial ROS (mtROS) and increase mitochondrial dynamics. Furthermore, in oocytes of obese mice, it has been shown to reverse meiotic defects and improve mitochondrial function (Wu et al., 2015; Ohlen et al., 2017; Sumegi et al., 2017). In the current study, we show that treatment of oocytes with either MitoQ or BGP-15 reverses the spindle and chromosomal abnormalities which occur during increased oxidative stress and in reproductively aged female mice. Importantly, MitoQ treatment during in-vitro culture significantly enhances maturation rates and reduces chromosomal defects in human oocytes.
Materials and methods
Mouse oocyte collection
The Animal Ethics Committee of Monash University approved all animal handling and experimental protocols, which were conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. C57BL/6J mice were obtained from Monash Animal Research Platform and housed under controlled environmental conditions with free access to water and food.
Germinal vesicle (GV)-stage oocytes were collected from the ovaries of 1-month-old pre-pubertal (referred to as young) or old (12, 15 or 18 month) C57BL/6J female mice that had been hormonally stimulated by intraperitoneal injection of 7 IU pregnant mare's serum gonadotropin (Intervet, Buckinghamshire, UK) 44–48 h prior. After removing adipose tissue surrounding the ovaries, cumulus–oocyte complexes (COC) containing fully grown GV-stage oocytes encircled by cumulus cells were released by puncturing the ovaries in M2 medium supplemented with 200 µM 3-isobutyl-1-methylxanthine (Sigma-Aldrich, St. Louis, MO, USA) to maintain GV arrest at 37°C. Attached cumulus cells were removed by repetitive pipetting of COCs. Denuded GV oocytes were cultured in either M2 medium at 37°C or in M16 medium (Sigma-Aldrich) at 37°C with 5% of CO2 in air as indicated in the respective figure legends. GV oocytes were cultured for 8 h to reach metaphase I (MI) or 14 h to reach metaphase II (MII) stage.
Collection and maturation of human oocytes
Human biological material was obtained in compliance with the National Statement on Ethical Conduct in Human Research (NHMRC, 2007) and was approved by the Monash Surgical Hospitals Human Research Ethics Committee. At the clinic, female patients were subjected to ovarian stimulation protocol, typically including GnRH (Orgalutran, 250 mg, Shering-Plough) and FSH (Puregon 100–375 U; Schering-Plough, North Ryde, NSW or Gonal F, 150–350 U Merck Serono, Frenchs Forest, NSW) as a preparation for IVF cycle. According to the standard/routine procedures and after observing 2–3 follicles of 18–20 mm diameter by ultrasound examination and reaching a level of 17 β-oestradiol concentration in the blood of 150–200 pg/ml/follicle over 18 mm, the patient would receive hCG. Oocytes were transvaginally aspirated ∼36 h after administration of 10 000 IU hCG (Ovidrel, 250 mg; Merck Serono). After retrieval, COCs were collected in a 90-mm Petri-dish (Vitrolife) and were washed through G-IVF+ (Vitrolife) before being placed into 1 ml of G-IVF+ in groups of four COCs. Cumulus cells were removed within 3–4 h after collection (39–40 h post-hCG) by a brief hyaluronidase exposure (Hyalase 25 IU/ml, Sanofi, Macquarie Park, NSW) with a fine up/down bore pipetting. Human oocytes that were to be discarded because they were at the GV stage and unsuitable for ICSI were provided by Monash IVF (Clayton, VIC, Australia), following informed consent of the patient. Immature oocytes from patients (aged 29–45 years) were obtained, who had varying diagnoses of infertility. Only oocytes that were morphologically normal (89 oocytes) were included in the experiments.
GV-stage oocytes were randomly assigned to the control group or the MitoQ-treated group. Oocytes were cultured in 20 µl drops of M199 medium (Sigma-Aldrich, St. Louis, MO, USA), with or without 50 nM MitoQ, overlayed by mineral oil (Sigma-Aldrich). Incubation was at 37°C with low oxygen (5% O2, 5% CO2 and 90% N2). After 30 h, oocytes were examined for polar body extrusion.
Measurement of mitochondrial membrane potential of mouse and human oocytes
Oocytes were labeled with 50 nM Tetramethylrhodamine, Methyl Ester, Perchlorate (TMRM) (Sigma-Aldrich) and MitoTracker Green (Thermo Fisher) in M2 medium for 30 min at 37°C. TMRM is a small cationic fluorescent indicator that accumulates in mitochondria purely based on mitochondrial membrane potential (MMP) and MitoTracker Green labels mitochondria independent of MMP. TMRM was excited using the 552 nm laser, and fluorescence collected using a 560–630 nm band pass filter. MitoTracker Green was excited using the 488 nm laser and fluorescence collected using a 495–540 nm band pass filter. Confocal sections obtained from the LASX software (Leica) were analyzed using ImageJ for measurements of MMP. Gray values were derived from the ‘analyze’ function of ImageJ to evaluate the fluorescent intensity of oocyte mitochondria. Ratio of TMRM and MitoTracker Green was calculated to measure the MMP. Values were normalized to the mean of control values in each experimental replicate to avoid the impact of day-to-day variations in the fluorescence ratio.
Determination of mtROS
mtROS were measured using Mitosox Red (Thermo Fisher Scientific, Waltham, MA, USA). Oocytes were incubated with 5 µM Mitosox in M2 media at 37°C for 30 min. Epifluorescence and bright field images were acquired using SP8 confocal microscope (Leica, Germany) and fluorescence was measured at an excitation wavelength of 488 and 520 nm long pass filter. Images were acquired using a 40× water immersion 1.2 NA objective with line averaging set to 2–4. Confocal sections obtained from the LAS AF Lite software were analyzed using ImageJ for measurements of MMP and ROS. Gray values were derived from the ‘analyze’ function of ImageJ to evaluate the fluorescent intensity of oocyte mitochondria.
Meiotic spindle analysis of mouse and human oocytes
After IVM, MII oocytes were fixed in 4% (w/v) paraformaldehyde (Sigma-Aldrich) and 2% (v/v) Triton X-100 (Sigma-Aldrich) in PBS for 40 min at 25°C. Oocytes were blocked in PBS containing 10% (w/v) BSA and 2% (v/v) Tween 20 for 1 h at 25°C and incubated with FITC-conjugated mouse anti-α-tubulin (1:200, Alexa Fluor 488, Thermo Fisher Scientific) for 1 h at 25°C. After three washes, chromosomes were labeled using Hoechst 33342 (10 µg/ml, Sigma-Aldrich) for 10 min and oocytes were mounted in PBS drops on glass-bottomed dishes (World Precision Instruments, Sarasota, FL, USA) for imaging. Serial Z sections of labeled oocytes were acquired using a laser-scanning confocal microscope imaging system (SP8; Leica) and 40× water immersion objective (1.2 NA) at 25°C. FITC was excited using the 488 nm laser and a band pass of green-fluorescent emission was 495–540 nm. Hoechst 33342 was excited with 405 nm laser and emission was collected with a band pass of 438–458 nm. Images were analyzed using Las X software (Leica). Imaris software (Bitplane AG, Switzerland) was used to orient 3-dimensional confocal stacks for measurement of spindle length and width.
Treatment of mouse oocytes with H2O2, BGP-15 and MitoQ
MII oocytes were exposed to 25 and 50 µM hydrogen peroxide (Merck, Germany) for 1 h at 37°C in M2 medium and control group of oocytes were cultured in M2 medium only for 1 h. These oocytes were fixed to label spindle and chromosomes. Twenty-five micromole of H2O2 was found as suitable dose to induce oxidative stress as it caused mild spindle defects and chromosome misalignment (Supplementary Fig. S1). In separate experiments, GV-stage oocytes were matured in vitro with 0, 25 and 50 µM H2O2 in M2 medium and maturation rates were scored after 14 h of culture. Twenty-five micromole of H2O2 did not significantly alter maturation rate (Supplementary Fig. S1). We tested two mitochondria-targeted compounds, MitoQ and BGP-15, which have been shown to have protective effects in several oxidative stress-related pathologies in human and animal studies (Wu et al., 2015; Murphy, 2016; Ohlen et al., 2017; Sumegi et al., 2017; Rossman et al., 2018). Concentrations used in previous studies, and dose optimization experiments measuring polar body extrusion (PBE) during IVM, indicated that 10 µM BGP-15 (Supplementary Fig. S2A) and 50 nM MitoQ (Supplementary Fig. S2B) were suitable doses to test in our oxidative stress and aging models. Oocytes from 1-month-old mice were cultured in various concentrations (0, 25, 50, 100 and 200 nM) of MitoQ (MitoQ limited, Auckland, New Zealand) or BGP-15 (Sigma-Aldrich) (0, 10, 50 and 100 µM) in M2 medium. Based on minimum effects on maturation rates and MII spindles-chromosome alignments, doses of 10 µM BGP-15 and 50 nM MitoQ were chosen to test in our oxidative stress and ageing models (Supplementary Fig. S2). These reagents were subsequently used during IVM of mouse and human oocytes as indicated in the figure legends.
Stock solutions of MitoQ (300 mM) and BGP-15 (10 mM) were prepared in deionized water and aliquots were stored at −20°C. Maximum dilution of culture medium was 0.5%.
In-vitro transcription and microinjection
Mito-GFP mRNA was transcribed using the T3 mMessage mMachine Kit (Ambion) according to the manufacturer’s protocol and resuspended in RNase-free water. mRNA (1.2 mg/ml) was delivered using a microinjection apparatus consisting of Narishige micromanipulators (Narishige, Japan) mounted on a Zeiss S100TV inverted microscope. A controlled delivery of ∼5% oocyte volume was delivered to GV stage-arrested oocytes using a picopump. After injection, oocytes were washed out of 3-isobutyl-1-methylxanthine (IBMX) and transferred in M16 medium. To label the endoplasmic reticulum, 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI, Sigma-Aldrich) was microinjected as a saturated solution in soybean oil (Sigma-Aldrich) 30 min prior to imaging. DiI was excited using the 552 nm laser, and fluorescence collected using a 560–630 nm band pass filter. Mito-GFP was excited using the 488 nm laser and fluorescence collected using a 495–540 nm band pass filter.
Statistical analysis
All experiments were repeated at least three times and mean and SEM were calculated. Statistical analysis was performed using either Student’s t-test or one-way ANOVA with Microsoft Excel or GraphPad Prism Software. Level of significance is denoted by *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 as shown in the figures.
Results
Spindle and mitochondrial defects in oocytes of aged mice
We initially established the effect of aging on mouse oocyte spindle organization during IVM. Immature GV oocytes were collected from 1-, 12- and 18-month-old female mice and matured in vitro. As expected, the number of GV-stage oocytes recovered from each mouse declined markedly with aging (20–25 oocytes from 1-month-old mice, 8–10 oocytes from 12-month-old mice and 1–3 oocytes from 18-month-old mice). Following culture in vitro, the rates of oocyte maturation as indicated by PBE were 83% (1 month), 76% (12 months) and only 50% in oocytes from 18-month-old mice (Fig. 1A). Spindle and chromosome alignment in oocytes were assessed in the in-vitro matured MII oocytes by labeling tubulin and DNA. We found that the percentage of MII-stage oocytes with misaligned chromosomes was significantly higher in 18-month-old mice (61%) than in oocytes from 1-month-old mice (20%) and 12-month-old mice (23%) (Fig. 1B and C).
Figure 1.
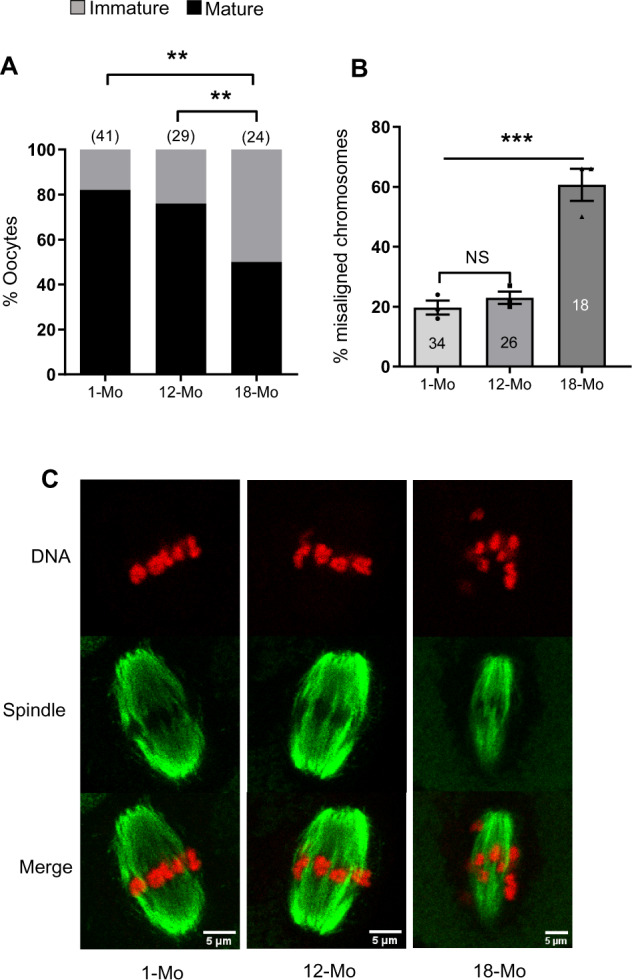
Maturation and chromosomal misalignment rates of oocytes isolated from mice at different ages. (A) Maturation rates after in-vitro culture. (B) Percentage of oocytes with one or more misaligned chromosomes. (C) Representative images of MII oocytes with labeled spindles (green) and DNA (red). Mo, month. Results are presented from at least three replicate experiments in all cases. Error bars show SE of the mean. Number of oocytes analyzed in each group are shown. **P ≤ 0.01; ***P ≤ 0.001 and NS, not significant.
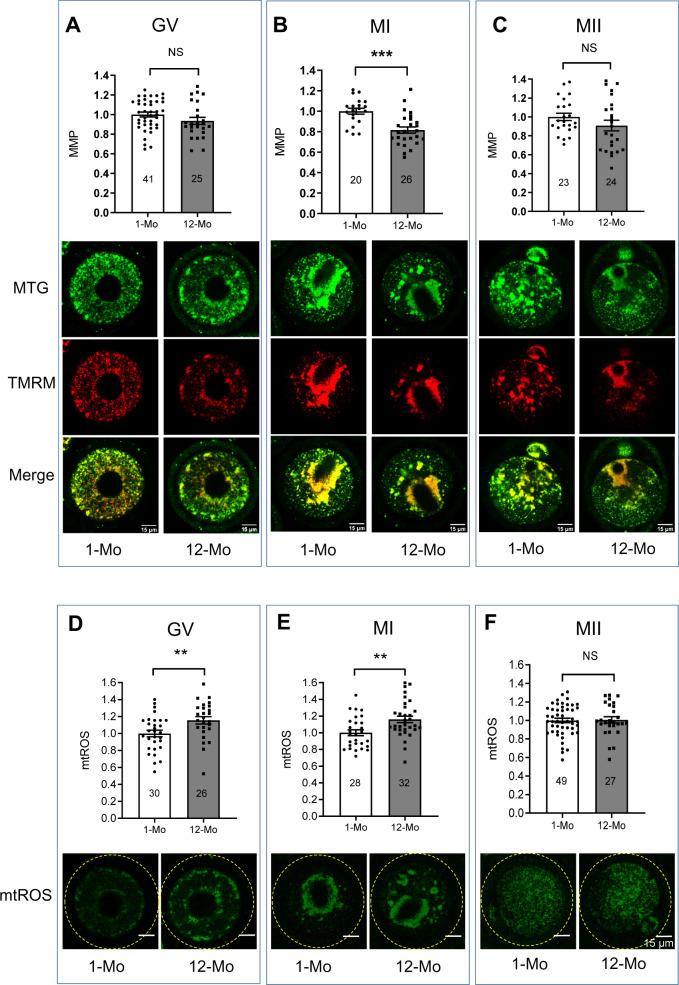
ROS generation may lead to mitochondrial damage that may decrease the MMP. Thus, MMP levels were compared between oocytes from 1- and 12-month-old females. At the GV (Fig. 2A) and MII (Fig. 2C) stages, MMP was comparable at all ages while at MI (Fig. 2B), MMP was significantly lower in oocytes from 12-month-old females compared to 1-month-old controls.
Figure 2.
Comparison of mitochondrial membrane potential and mitochondrial reactive oxygen species (ROS) during oocyte maturation. Germinal vesicle, and metaphase I and II (GV, MI and MII) stage oocytes were labeled with Tetramethylrhodamine, Methyl Ester, Perchlorate (TMRM) and mitotracker green (MTG) and mitochondrial membrane potential (MMP) was calculated as the ratio of TMRM and MTG. (A) MMP comparison in GV-stage oocytes. (B) MMP comparison in MI-stage oocytes. (C) MMP comparison in MII-stage oocytes. Corresponding representative images of oocytes labeled with MTG (green), TMRM (red) and merged (yellow) are shown under each figure. GV, MI and MII oocytes from young and 12-month-old mice were labeled with MitoSOX red. (D) Comparison of ROS in GV-stage oocytes. (E) Comparison of ROS in MI-stage oocytes. (F) Comparison of ROS in MII-stage oocytes. Representative images of MitoSOX-labeled (green) oocytes are shown below each panel. Error bars show SE of the mean. Number of oocytes analyzed in each group are shown. **P ≤ 0.01; ***P ≤ 0.001 and NS, not significant.
To measure mtROS levels in immature and mature oocytes from young and older female mice, oocytes were collected at the GV stage and labeled with MitoSOX Red or matured in vitro and labeled at MI (8 h) and MII (14 h). Due to the low numbers of oocytes available at 18 months of age, MitoSOX measurements were limited to oocytes from mice of 1 and 12 months of age. mtROS levels at the GV (Fig. 2D) and MI (Fig. 2E) stages were significantly higher in oocytes from 12-month-old mice compared to oocytes from young mice. However, at the MII stage, mtROS levels were similar in oocytes from young and 12-month-old females (Fig. 2F).
Since the GV-stage oocytes from 12-month-old females did not show significant reduction in MMP compared to young control oocytes, we tested if MMP was affected when mice were 15 and 18 months of age. Consistent with the timing of chromosomal misalignment, MMP levels in 12- and 15-month-old oocytes were similar to young controls, but at 18 months, we observed a significant decrease in MMP (Supplementary Fig. S3). Interestingly these effects on mitochondrial function appear to precede the timing of spindle disruption, which in our system, was only detected in oocytes from 18-month-old mice.
Because the oocytes from older mice exhibited increased mtROS and decreased MMP, and others have also made the correlation with spindle disruption (Pasquariello et al., 2019), we have asked if oxidative stress in young oocytes is sufficient to induce spindle defects as seen in old oocytes. An initial experiment revealed that H2O2 exposure at the GV stage did not impact the subsequent movement of mitochondria to the spindle region (Supplementary Fig. S1A). Treatment of MII oocytes from young mice with 25 µM H2O2 for 1 h significantly increased the percentage of oocytes with misaligned chromosomes (61%) compared to untreated control oocytes (20%) (Fig. 3 and Supplementary Fig. S1B). However, we did not find a significant difference in spindle length between control and H2O2-treated oocytes (not shown). Interestingly, the rates of chromosomal misalignment caused by H2O2 treatment in young oocytes (61%) were comparable to the rates of chromosomal misalignments observed in oocytes from 18-month-old mice (63%) (Fig. 3A and B). This oxidative stress test with 25 µM H2O2 is therefore sufficient to induce spindle disruption, and provides a useful model system for testing potential mitochondrial therapeutics.
Figure 3.
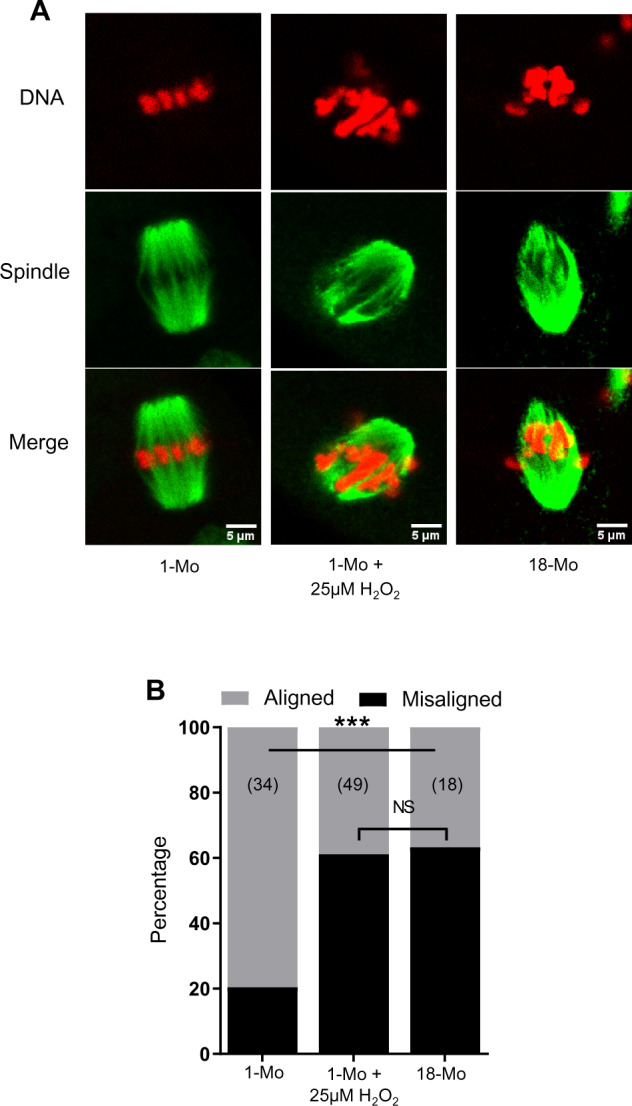
Generation of reactive oxygen species (ROS)-induced model of spindle and chromosomal defects. Germinal vesicle (GV) stage oocytes were collected from 1 month (1-Mo) and 18-Mo-old mice and were matured in vitro in M2 medium. (A) Metaphase II (MII) oocytes in 1-Mo group either remained in M2 medium or were treated with 25 µM H2O2 for 1 h. The 18-Mo group MII oocytes remained untreated. Mature oocytes were fixed and labeled for spindles (green) and DNA (red). (B) Percentage of oocytes containing aligned or misaligned chromosomes in different groups. Number of oocytes analyzed in each group are shown. ***P ≤ 0.001 and NS, not significant.
BGP-15 and MitoQ improve MMP in oocytes undergoing oxidative stress or aging
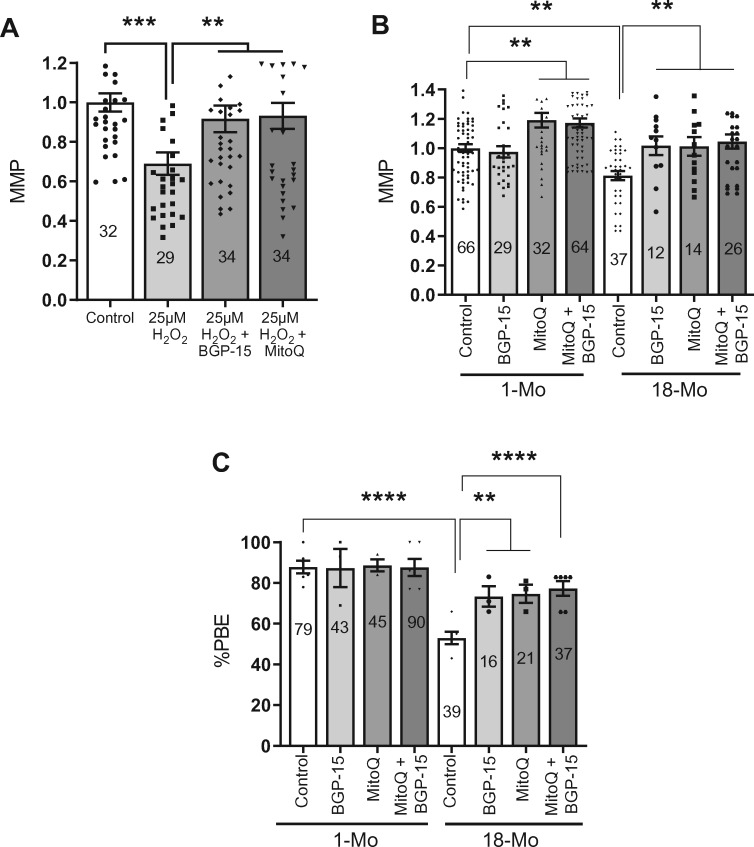
To examine the effect of MitoQ and BGP-15 in our oxidative stress model, in-vitro matured MII oocytes from young mice were exposed to 25 µM H2O2 in the presence or absence of BGP-15 or MitoQ. Consistent with experiments outlined above, control oocytes stressed with 25 µM H2O2, showed a significantly reduced MMP, which was completely prevented in oocytes matured in the presence of BGP-15 or MitoQ (Fig. 4A).
Figure 4.
Enhanced mitochondrial membrane potential (MMP) and oocyte maturation rates by mitochondria-targeted agents. (A) H2O2 significantly decreased MMP but BGP-15 or MitoQ can prevent such decrease in MMP in metaphase II (MII) oocytes from 1 month (1-Mo) old mice. (B) Comparison MMP in MII oocytes from young (1-Mo) and old (18-Mo) mice after IVM in the absence or presence of BGP-15, MitoQ and both combined. Number of oocytes analyzed in each group are shown. (C) Comparison of maturation rates of oocytes from young (1-Mo) and old (18-Mo) mice after in-vitro culture in the absence or presence of BGP-15, MitoQ and both combined. Error bars show SE of the mean. Number of oocytes analyzed in each group are shown. **P ≤ 0.01, ****P ≤ 0.0001.
We then turned to address oocytes from old mice and whether MitoQ and BGP-15 could improve MMP and the rate of PBE. Oocytes from 1- and 18-month-old mice were cultured without any agent (controls) or in the presence of BGP-15 or MitoQ, or a combination of both. We found that BGP-15 treatment did not significantly affect MMP in oocytes from young mice but it significantly elevated MMP levels in oocytes from 18-month-old mice (Fig. 4B and Supplementary Fig. S4). The presence of MitoQ during IVM resulted in MII-stage oocytes with a significantly increased MMP in both the young and old mouse oocytes compared to their untreated controls (Fig. 4B and Supplementary Fig. S4). We did not observe any additive or synergistic effect on MMP when oocytes were matured in the presence of BGP-15 and MitoQ (Fig. 4B).
Importantly, we also observed that BGP-15 or MitoQ significantly boosted PBE rates in oocytes from old mice (Fig. 4C). However, these agents did not further enhance the PBE rates in the oocytes from young mice (Fig. 4C). These results show that mitochondria-targeted antioxidants can enhance MMP and oocyte maturation, particularly in oocytes from reproductively older females.
Reversal of ROS-mediated or age-mediated spindle and chromosome defects by mitochondria-targeted agents
We next examined whether MitoQ and BGP-15 can rescue the spindle and chromosomal defects induced by H2O2. Consistent with data in Fig. 3, treatment of oocytes from young mice with H2O2 results in a marked increase in misaligned chromosomes compared to untreated controls (63% versus 20%; Fig. 5A–C). This increase in oocytes displaying misaligned chromosomes is significantly reduced in BGP-15-treated (31%) and abolished in MitoQ-treated oocytes (20%). A similar pattern of results is seen when counting the number of misaligned chromosomes. Oocytes treated with H2O2 show an average of approximately two misaligned chromosomes compared to less than one in controls and MitoQ or BGP-15-treated oocytes (Fig. 5C).
Figure 5.
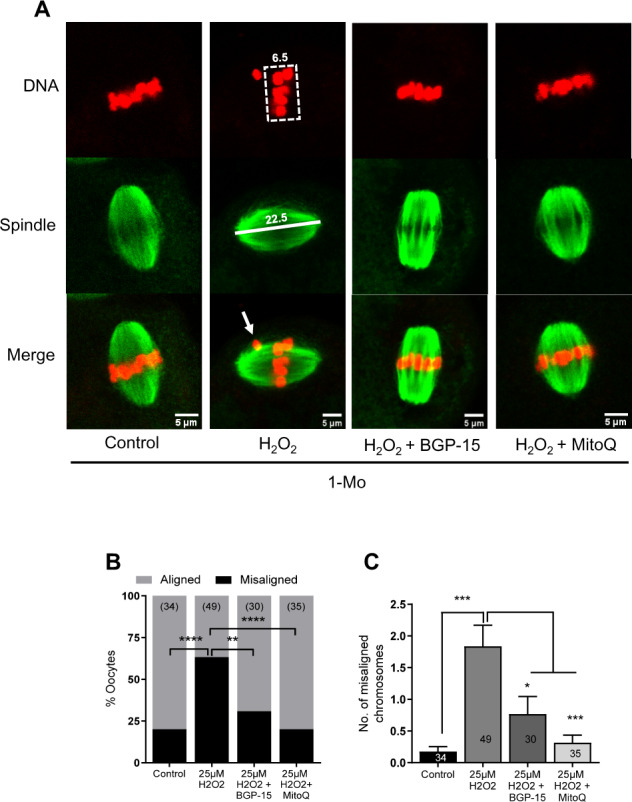
Rescue of chromosomal misalignments induced by oxidative stress in young mouse oocytes. (A) Germinal vesicle (GV) stage oocytes collected from 1 month (1-Mo) old mice were matured in M2 medium only (control) or in the presence of 10 µM BGP-15 or 50 nM MitoQ. Metaphase II (MII) oocytes were treated with 25 µM H2O2 for 1 h and were fixed and labeled for spindles (green) and DNA (red). (B) Percentages of oocytes with aligned or misaligned chromosomes. (C) Quantification of average number of misaligned chromosomes per oocyte in different treatment groups. Error bars show SE of the mean. Number of oocytes analyzed in each group are shown. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
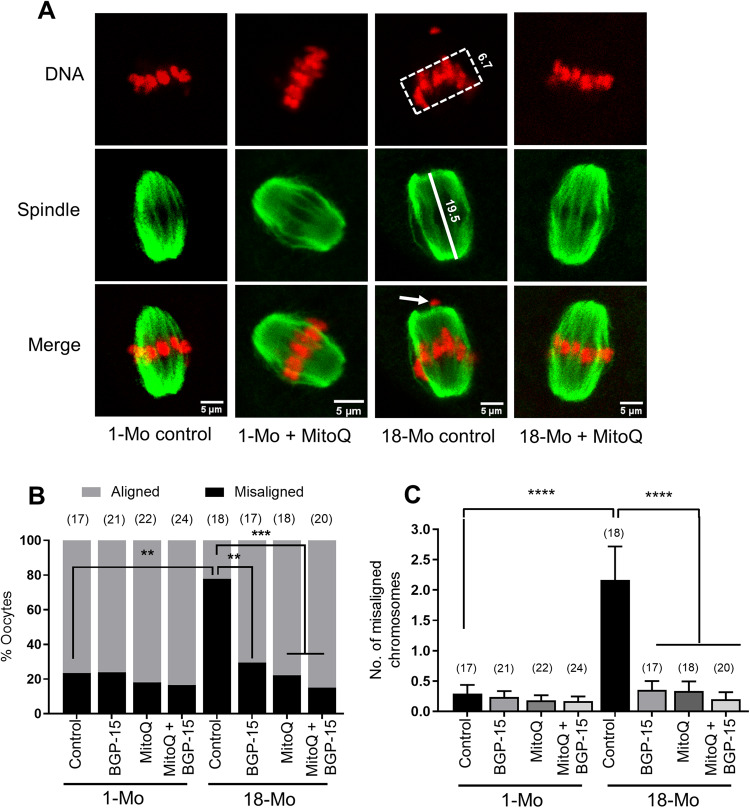
We next set out to test if BGP-15 and MitoQ can also reverse the chromosomal misalignment seen after IVM of oocytes from old mice. We found that about 78% of oocytes from 18-month-old mice displayed chromosomal misalignments (Fig. 6A and B), which was significantly reduced by addition of BGP-15 (29%, Fig. 6B), and completely reversed after treatment with MitoQ (22%, Fig. 6A and B) or both combined (15%, Fig. 6B). No effect of any of the treatments was seen on oocytes isolated from young mice (Fig. 6A and B). Similarly, the number of misaligned chromosomes per oocyte from old females (2.16) was reduced to control levels (0.3) after treatment with BGP-15 (0.35), MitoQ (0.33) or both (0.2) (Fig. 6C). Interestingly, while oxidative stress or aging disrupted spindle organization of chromosomes, the average spindle length did not differ compared to controls and was also not impacted by MitoQ or BGP-15. Together, our data show that BGP-15 and MitoQ treatment during oocyte maturation can reverse the effects of maternal aging and oxidative stress on spindle organization and chromosome misalignment.
Figure 6.
Rescue of chromosomal misalignments in oocytes isolated from aged mice. (A) Germinal vesicle (GV) stage oocytes from 1-month-old and 18-month-old mice were matured in M16 medium only (control) or with 10 µM BGP-15, 50 nM MitoQ or BGP-15 + MitoQ at 37°C with 5% CO2. Mature oocytes were fixed and labeled for spindles and DNA. Representative MII spindle and DNA in control oocyte from 1-Mo-old mice, 1-Mo MitoQ treated, 18-Mo control and 18-Mo MitoQ treated are shown. (B) Percentages of oocytes with aligned or misaligned chromosomes. (C) Quantification of average number of misaligned chromosomes per oocyte. Error bars show SE of the mean. Number of oocytes analyzed in each group are shown. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
MitoQ improves IVM of human oocytes
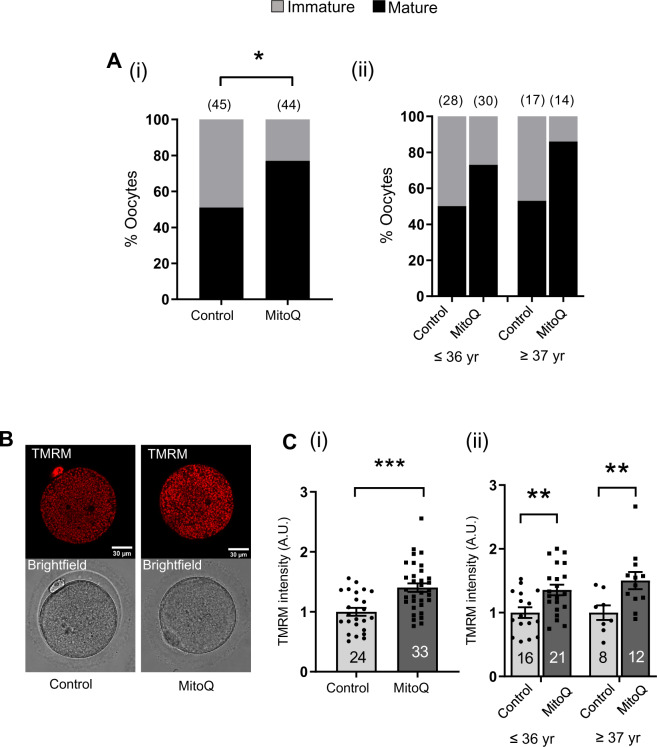
Our results indicate that MitoQ and BGP-15 during maturation can significantly improve the quality of mouse oocytes isolated from old mice. The inherent molecular differences make humans eggs more prone to aneuploidy than oocytes of many other species (Schuh and Ellenberg, 2007; Holubcová et al., 2015). We next sought to further pursue and examine the effectiveness of MitoQ for the improvement of spindle and chromosome alignment in human oocytes. Eighty-nine GV-stage human oocytes were randomly assigned to undergo maturation in control conditions or in the presence of 50 nM MitoQ. The average age of patients was 36.0 ± 4.8 years in control and 35.7 ± 4.4 years in the MitoQ treatment group. After IVM for a period of 30 h, 51% (n = 45) of oocytes cultured in control medium extruded PB1. This rate of maturation was dramatically improved to 77% (n = 44) in the presence of MitoQ (Fig. 7Ai). We analyzed the maturation rate broken down by maternal age and found that MitoQ similarly improved maturation across all age groups examined (Fig. 7Aii).
Figure 7.
IVM of human oocytes in the presence of MitoQ. (A) Comparison of maturation rates between control and 50 nM MitoQ-treated groups (i). Data are expressed as percentages and chi-square test was used to compare between groups, *P < 0.05. (ii) Comparison of maturation rates between control and MitoQ-treated oocytes from (i) in different age groups. (B) Representative Tetramethylrhodamine, Methyl Ester, Perchlorate (TMRM) fluorescence and bright field images. (C) Quantification of TMRM signal intensities between control and MitoQ-treated metaphase II (MII) oocytes (i). (ii) Comparison of TMRM signal intensities between control and MitoQ-treated oocytes from (i) in different age groups. Data are expressed as mean ± SEM. Chi-square test (A) or Student’s t-test (C) was used to compare between groups.; *P < 0.05, **P < 0.01 and ***P < 0.001.
MII-stage oocytes matured in the absence (control) or presence of MitoQ were labeled with TMRM to assess the effect of MitoQ on MMP before they were processed for spindle and DNA analysis by immunofluorescence. Analysis of TMRM intensity revealed that oocytes cultured with MitoQ had significantly higher TMRM intensity compared to controls; an effect that was seen across all age ranges (Fig. 7B and C).
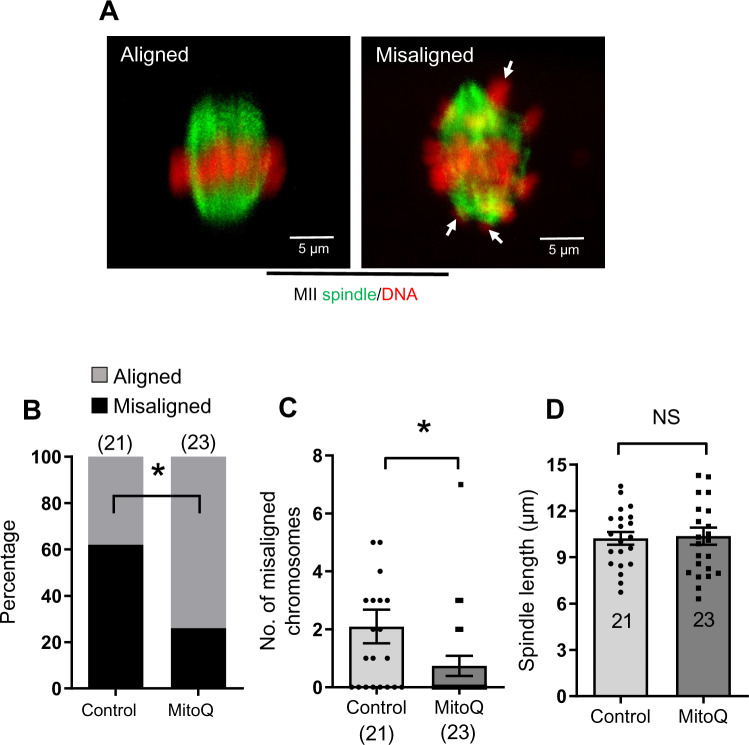
MitoQ treatment significantly decreased the percentage of oocytes with misaligned chromosomes compared to control groups (25% versus 61%, respectively) (Fig. 8A and B). On average about two chromosomes per oocyte were misaligned upon maturation without MitoQ but this number was significantly decreased after MitoQ treatment (Fig. 8C). However, MitoQ treatment did not significantly affect the length of the spindle (Fig. 8D). These results altogether show that similar to mouse oocytes, MitoQ can significantly improve chromosome alignment in human oocytes.
Figure 8.
Spindle and chromosome alignments in in-vitro matured human oocytes in the presence of MitoQ. (A) Examples of aligned and misaligned chromosomes (arrows). Metaphase II spindles and chromosomes were visualized by staining tubulin and DNA to analyze chromosomal misalignment. (B) Percentage of mature oocytes with aligned and misaligned chromosomes in control and MitoQ groups. (C) Number of misaligned chromosomes per oocyte in control and MitoQ groups. (D) Comparison of spindle length in control and MitoQ groups. Error bars show SE of the mean. Number of oocytes analyzed in each group are shown. Chi-square test (B) or Student’s t-test (C and D) was used to compare between groups. *P < 0.05, NS, not significant.
Discussion
In this study, we show that IVM in the presence of mitochondria-targeted therapeutics, MitoQ and BGP-15, ameliorate the spindle abnormalities and chromosomal misalignments in eggs from aged mice. Spindle and chromosomal abnormalities in eggs are the known causes of defective embryogenesis, high rates of miscarriage and infertility in humans. We also show that MitoQ treatment during IVM in human oocytes can significantly improve the maturation rates and spindle-chromosome alignment, which together indicate their potential for application in the clinic.
Mitochondrial defects have been implicated in poor oocyte quality and infertility in various maternal conditions such as aging and obesity (Grindler and Moley, 2013; Ben-Meir et al., 2015; Wu et al., 2015; Babayev et al., 2016; Pasquariello et al., 2019; Seli et al., 2019). One of the main biochemical consequences of aging is increased ROS generation due to mitochondrial malfunction (Liu et al., 2012; Boots et al., 2016; Mihalas et al., 2017). Therefore, ROS reduction by antioxidants presents a potential therapeutic means of treating maternal aging-associated mitochondrial defects in oocytes (Liu et al., 2012; Bentov et al., 2014; Ben-Meir et al., 2015; Marei et al., 2019). Improving mitochondrial function is one of the potential strategies to enhance oocyte quality and reproductive success.
MMP is often used as a proxy for mitochondrial function. We, like others, find significant variation between oocytes at any one stage of development which means it is challenging to confidently assign biological effects to relatively small changes in the average MMP. With this caveat in mind, here we find MI is the only stage that shows an age-associated decrease in MMP. Interestingly, our previous studies directly comparing MMP between the GV and MI stage show that MMP peaks at the MI stage (Al-Zubaidi et al., 2019), raising the possibility that old oocytes fail to increase MMP to the same extent between GV and MI stages, at least in this IVM system.
The reduced MMP in old MI oocytes is mirrored by a significant increase in mtROS but it is unclear if it is a causal relationship because it does not hold at the GV stage where mtROS is increased but MMP is unaffected by age. In MII-stage oocytes, there is no effect of age on MMP or ROS, a situation that may be explained if progression beyond MI represents a hurdle only achieved by those best functioning oocytes. There is clearly more to be done to understand the relationship between MMP and ROS in the context of aging and meiotic progression, especially given ROS and redox state are known to change with cell cycle state in other systems (Burhans and Heintz, 2009).
The increased ROS and reduced MMP in 12-month-old MI oocytes may contribute to the spindle and chromosomal abnormalities associated with maternal aging. However, given we only observed spindle defects in 18-month-old mice, if such a relationship does exist, it may be that the MMP-ROS axis is exacerbated by 18 months of age or that spindle and chromosome organization is a result of cumulative damage during maternal aging.
A role for ROS is supported by the findings that oxidative stress via exposure of oocytes from reproductively young mice to H2O2 is sufficient to induce spindle abnormalities and chromosome misalignments in a manner that is rescuable by using MitoQ and BGP-15. Given we find that mtROS is elevated at the GV stage in old oocytes, these studies indicate that it may be necessary to therapeutically target mitochondrial dysfunction in GV-stage oocytes in order to have the best chance of avoiding spindle damage.
The fact that the oxidative stress model phenocopies the chromosome and spindle defects observed in oocytes from reproductively aged mice, which are also fully rescued by MitoQ and BGP-15, is further support that oxidative stress contributes to the aging oocyte phenotype. A number of studies have investigated the effects of non-targeted, cytoplasmic antioxidants on oocyte quality of reproductively old or obese mice with generally positive results (Bentov et al., 2010, 2014; Liu et al., 2013; Ben-Meir et al., 2015; Boots et al., 2016). However, directly targeting the source of ROS using mitochondria-targeted molecules, such as MitoQ and BGP-15, is clearly highly effective in restoring MMP as well as spindle function and chromosome alignment in oocytes from old mice.
Both MitoQ and BGP-15 have long and impressive records of being effective in other contexts of cellular stress. MitoQ is a ubiquinone moiety linked to a lipophilic triphenylphosphonium cation, which is responsible for mitochondrial localization, and its ubiquinol form acts as an antioxidant. It acts as an antioxidant by becoming oxidized to a ubiquinone, which is recycled back to its active form, ubiquinol, by complex II (James et al., 2005). MitoQ has been found to protect against oxidative damage in many different tissues in rodents (Murphy, 2016) and it has been shown to be safe to use in humans (Gane et al., 2010; Snow et al., 2010) and has been shown to be effective in treating age-associated hypertension (Rossman et al., 2018).
MitoQ is water soluble, orally active and accumulates intracellularly. MitoQ has been effective in preventing ischemia reperfusion-induced cardiac dysfunction and improves vascular functions (Rossman et al., 2018), improves memory and extends lifespan in mice (Young and Franklin, 2019), ameliorates irradiation-induced testicular damage in rats (Ibrahim et al., 2019) and renoprotection in diabetes (Ward et al., 2017). It has also been shown to protect against lipotoxicity in bovine oocytes (Marei et al., 2019).
BGP-15 is a hydroxylamine derivative and while a definitive mechanism of action or target protein has not yet been identified, it has been shown to preferentially accumulate in mitochondria (Sumegi et al., 2017) and has undergone clinical trials in humans (Literáti-Nagy et al., 2009) where it is orally administered. We have previously shown it to be beneficial in improving oocyte and embryo quality in obese mice (Wu et al., 2015). BGP-15 has also been found to improve neuronal functions (Ohlen et al., 2017), muscle function (Gehrig et al., 2012; Nascimento et al., 2018) and cardiac function (Szabados et al., 2000; Bombicz et al., 2019) in different disease models. Despite all this evidence for protecting against the effects of aging and oxidative stress in other systems, a role for MitoQ and BGP-15 in rescuing aging-related spindle and chromosomal abnormalities in oocytes had remained unknown.
With the encouraging results obtained from mouse experiments, we examined if MitoQ treatment is beneficial during human oocyte maturation in vitro. The oocytes we used were those that were found to be immature at the time of denudation for ICSI. As with all experiments using these so-called ‘ICSI-GV’ oocytes, our results need to be interpreted with some caution. Nevertheless, these oocytes underwent maturation to metaphase II at relatively high rates and as such, their compromised state provided an ideal model system to test if MitoQ could improve spindle function in this somewhat rudimentary IVM system.
As expected, over 60% of control human oocytes displayed chromosomal misalignment in MII oocytes, which was reduced by more than half to only about 26% when the oocytes were cultured in the presence of MitoQ. This treatment was also accompanied by significant improvement in the rates of oocyte maturation from 51% to 77%. Importantly, although oocyte aneuploidy increases dramatically in the late 30 s and early 40 s (Hassold et al., 2007; Nagaoka et al., 2012), meiosis in humans across all ages appears to be highly error-prone (Holubcová et al., 2015). Thus, MitoQ could be clinically useful in contexts other than aging.
Given that increasing numbers of women delay childbearing, there is an imperative to understand how to improve fertility and reduce miscarriage and chromosomal anomalies associated with maternal aging. Our study identifies two excellent candidates that may help to improve fertility in older women. Importantly, these potential therapies must be tested for efficacy in clinical IVM systems and undergo thorough examination of resultant offspring in preclinical models before utilization. However, our results using in-vitro systems for oocyte maturation in both mouse and human provide proof of principle that mitochondrially targeted molecules such as MitoQ and BGP-15 may represent a novel therapeutic approach against maternal aging-related spindle and chromosomal abnormalities.
Supplementary data
Supplementary data are available at Human Reproduction online.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Supplementary Material
Acknowledgments
The authors thank Jeff Mann for comments and Monash Micro Imaging for support with confocal imaging. The authors gratefully acknowledge the staff of Monash IVF who assisted with the study, particularly Dr Jayne Mullin Scientific Director, and the patient participants of Monash IVF.
Authors’ roles
All authors qualify for authorship by contributing substantially to this article. U.A.-Z., D.A., R.L.R. and J.C. developed the original concept of this study collectively and wrote the manuscript. L.R. supported from Monash IVF. U.A.-Z., O.C., Q.-H.Z., W.S.Y. and D.A. collected data and U.A.-Z. performed statistical analysis. All authors have contributed to critical discussion, reviewed the final version of the article and approved it for publication.
Funding
The National Health and Medical Research Council and Australian Research Council, Australia financially supported the project. U.A.-Z. was supported by the Iraqi Higher Education and Scientific Research Ministry PhD scholarship and O.C. was supported by TUBITAK-1059B191601275.
Conflict of interest
M.P.M. consults for MitoQ Inc. and holds patents in mitochondria-targeted therapies. R.L.R. is an inventor on patents relating to the use of BGP-15 to improve gamete quality.
References
- Al-Zubaidi U, Liu J, Cinar O, Robker RL, Adhikari D, Carroll J.. The spatio-temporal dynamics of mitochondrial membrane potential during oocyte maturation. Mol Hum Reprod 2019;25:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angell RR. Aneuploidy in older women. Higher rates of aneuploidy in oocytes from older women. Hum Reprod 1994;9:1199–1200. [DOI] [PubMed] [Google Scholar]
- Babayev E, Wang T, Szigeti-Buck K, Lowther K, Taylor HS, Horvath T, Seli E.. Reproductive aging is associated with changes in oocyte mitochondrial dynamics, function, and mtDNA quantity. Maturitas 2016;93:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird DT, Collins J, Egozcue J, Evers LH, Gianaroli L, Leridon H, Sunde A, Templeton A, Van Steirteghem A, Cohen J. et al. ; ESHRE Capri Workshop Group. Fertility and ageing. Hum Reprod Update 2005;11:261–276. [DOI] [PubMed] [Google Scholar]
- Ben-Meir A, Burstein E, Borrego-Alvarez A, Chong J, Wong E, Yavorska T, Naranian T, Chi M, Wang Y, Bentov Y. et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 2015;14:887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentov Y, Esfandiari N, Burstein E, Casper RF.. The use of mitochondrial nutrients to improve the outcome of infertility treatment in older patients. Fertil Steril 2010;93:272–275. [DOI] [PubMed] [Google Scholar]
- Bentov Y, Hannam T, Jurisicova A, Esfandiari N, Casper RF.. Coenzyme Q10 supplementation and oocyte aneuploidy in women undergoing IVF-ICSI treatment. Clin Med Insights Reprod Health 2014;8:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoldo MJ, Listijono DR, Ho W-HJ, Riepsamen AH, Goss DM, Richani D, Jin XL, Mahbub S, Campbell JM, Habibalahi A. et al. NAD(+) repletion rescues female fertility during reproductive aging. Cell Rep 2020;30:1670–1681.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombicz M, Priksz D, Gesztelyi R, Kiss R, Hollos N, Varga B, Nemeth J, Toth A, Papp Z, Szilvassy Z. et al. The drug candidate BGP-15 delays the onset of diastolic dysfunction in the goto-kakizaki rat model of diabetic cardiomyopathy. Molecules 2019;24:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots CE, Boudoures A, Zhang W, Drury A, Moley KH.. Obesity-induced oocyte mitochondrial defects are partially prevented and rescued by supplementation with co-enzyme Q10 in a mouse model. Hum Reprod 2016;31:2090–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burhans WC, Heintz NH.. The cell cycle is a redox cycle: linking phase-specific targets to cell fate. Free Radic Biol Med 2009;47:1282–1293. [DOI] [PubMed] [Google Scholar]
- Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA.. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol 2010;20:1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton CM, Szabadkai G, Carroll J.. Measurement of ATP in single oocytes: impact of maturation and cumulus cells on levels and consumption. J Cell Physiol 2014;229:353–361. [DOI] [PubMed] [Google Scholar]
- Gane EJ, Weilert F, Orr DW, Keogh GF, Gibson M, Lockhart MM, Frampton CM, Taylor KM, Smith RA, Murphy MP.. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int 2010;30:1019–1026. [DOI] [PubMed] [Google Scholar]
- Gehrig SM, van der Poel C, Sayer TA, Schertzer JD, Henstridge DC, Church JE, Lamon S, Russell AP, Davies KE, Febbraio MA. et al. Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature 2012;484:394–398. [DOI] [PubMed] [Google Scholar]
- Greaney J, Wei Z, Homer H.. Regulation of chromosome segregation in oocytes and the cellular basis for female meiotic errors. Hum Reprod Update 2018;24:135–161. [DOI] [PubMed] [Google Scholar]
- Grindler NM, Moley KH.. Maternal obesity, infertility and mitochondrial dysfunction: potential mechanisms emerging from mouse model systems. Mol Hum Reprod 2013;19:486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassold T, Hall H, Hunt P.. The origin of human aneuploidy: where we have been, where we are going. Hum Mol Genet 2007;16:R203–R208. [DOI] [PubMed] [Google Scholar]
- Holubcová Z, Blayney M, Elder K, Schuh M.. Human oocytes. Error-prone chromosome-mediated spindle assembly favors chromosome segregation defects in human oocytes. Science 2015;348:1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AA, Karam HM, Shaaban EA, Safar MM, El-Yamany MF.. MitoQ ameliorates testicular damage induced by gamma irradiation in rats: modulation of mitochondrial apoptosis and steroidogenesis. Life Sci 2019;232:116655. [DOI] [PubMed] [Google Scholar]
- James AM, Cocheme HM, Smith RA, Murphy MP.. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J Biol Chem 2005;280:21295–21312. [DOI] [PubMed] [Google Scholar]
- Jones KT, Lane SI.. Molecular causes of aneuploidy in mammalian eggs. Development 2013;140:3719–3730. [DOI] [PubMed] [Google Scholar]
- Kitajima TS. Mechanisms of kinetochore-microtubule attachment errors in mammalian oocytes. Develop Growth Differ 2018;60:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SIR, Jones KT.. Non-canonical function of spindle assembly checkpoint proteins after APC activation reduces aneuploidy in mouse oocytes. Nat Commun 2014;5:3444. [DOI] [PubMed] [Google Scholar]
- Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, Nathan A, Floros V, Adelfalk C, Watanabe Y. et al. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol 2010;20:1511–1521. [DOI] [PubMed] [Google Scholar]
- Literáti-Nagy B, Kulcsár E, Literáti-Nagy Z, Buday B, Péterfai E, Horváth T, Tory K, Kolonics A, Fleming A, Mandl J. et al. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: a proof of concept randomized double-blind clinical trial. Horm Metab Res 2009;41:374–380. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu M, Ye X, Liu K, Huang J, Wang L, Ji G, Liu N, Tang X, Baltz JM. et al. Delay in oocyte aging in mice by the antioxidant N-acetyl-L-cysteine (NAC). Hum Reprod 2012;27:1411–1420. [DOI] [PubMed] [Google Scholar]
- Liu M, Yin Y, Ye X, Zeng M, Zhao Q, Keefe DL, Liu L.. Resveratrol protects against age-associated infertility in mice. Hum Reprod 2013;28:707–717. [DOI] [PubMed] [Google Scholar]
- Marangos P, Stevense M, Niaka K, Lagoudaki M, Nabti I, Jessberger R, Carroll J.. DNA damage-induced metaphase I arrest is mediated by the spindle assembly checkpoint and maternal age. Nat Commun 2015;6:8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marei WFA, Van den Bosch L, Pintelon I, Mohey-Elsaeed O, Bols PEJ, Leroy J.. Mitochondria-targeted therapy rescues development and quality of embryos derived from oocytes matured under oxidative stress conditions: a bovine in vitro model. Hum Reprod 2019;34:1984–1998. [DOI] [PubMed] [Google Scholar]
- Mihalas BP, De Iuliis GN, Redgrove KA, McLaughlin EA, Nixon B.. The lipid peroxidation product 4-hydroxynonenal contributes to oxidative stress-mediated deterioration of the ageing oocyte. Sci Rep 2017;7:6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP. Understanding and preventing mitochondrial oxidative damage. Biochem Soc Trans 2016;44:1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, Smith RA.. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol 2007;47:629–656. [DOI] [PubMed] [Google Scholar]
- Nabti I, Grimes R, Sarna H, Marangos P, Carroll J.. Maternal age-dependent APC/C-mediated decrease in securin causes premature sister chromatid separation in meiosis II. Nat Commun 2017;8:15346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka SI, Hassold TJ, Hunt PA.. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet 2012;13:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, FitzHarris G.. Intrinsically defective microtubule dynamics contribute to age-related chromosome segregation errors in mouse oocyte meiosis-I. Curr Biol 2017;27:1040–1047. [DOI] [PubMed] [Google Scholar]
- Nascimento TL, Silva MT, Miyabara EH.. BGP-15 improves contractile function of regenerating soleus muscle. J Muscle Res Cell Motil 2018;39:25–34. [DOI] [PubMed] [Google Scholar]
- Ohlen SB, Russell ML, Brownstein MJ, Lefcort F.. BGP-15 prevents the death of neurons in a mouse model of familial dysautonomia. Proc Natl Acad Sci USA 2017;114:5035–5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyewole AO, Birch-Machin MA.. Mitochondria-targeted antioxidants. FASEB J 2015;29:4766–4771. [DOI] [PubMed] [Google Scholar]
- Pasquariello R, Ermisch AF, Silva E, McCormick S, Logsdon D, Barfield JP, Schoolcraft WB, Krisher RL.. Alterations in oocyte mitochondrial number and function are related to spindle defects and occur with maternal aging in mice and humans. Biol Reprod 2019;100:971–981. [DOI] [PubMed] [Google Scholar]
- Pellestor F, Andréo B, Arnal F, Humeau C, Demaille J.. Maternal aging and chromosomal abnormalities: new data drawn from in vitro unfertilized human oocytes. Hum Genet 2003;112:195–203. [DOI] [PubMed] [Google Scholar]
- Rossman MJ, Santos-Parker JR, Steward CAC, Bispham NZ, Cuevas LM, Rosenberg HL, Woodward KA, Chonchol M, Gioscia-Ryan RA, Murphy MP. et al. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 2018;71:1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer MV. Reproduction at an advanced maternal age and maternal health. Fertil Steril 2015;103:1136–1143. [DOI] [PubMed] [Google Scholar]
- Schatten H, Sun Q-Y.. Centrosome and microtubule functions and dysfunctions in meiosis: implications for age-related infertility and developmental disorders. Reprod Fertil Dev 2015;27:934–943. [DOI] [PubMed] [Google Scholar]
- Schuh M, Ellenberg J.. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell 2007;130:484–498. [DOI] [PubMed] [Google Scholar]
- Seli E, Wang T, Horvath TL.. Mitochondrial unfolded protein response: a stress response with implications for fertility and reproductive aging. Fertil Steril 2019;111:197–204. [DOI] [PubMed] [Google Scholar]
- Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O'Sullivan JD, Fung V, Smith RA, Murphy MP, Taylor KM.. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson's disease. Mov Disord 2010;25:1670–1674. [DOI] [PubMed] [Google Scholar]
- Sumegi K, Fekete K, Antus C, Debreceni B, Hocsak E, Gallyas F Jr, Sumegi B, Szabo A.. BGP-15 protects against oxidative stress- or lipopolysaccharide-induced mitochondrial destabilization and reduces mitochondrial production of reactive oxygen species. PLoS One 2017;12:e0169372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabados E, Literati-Nagy P, Farkas B, Sumegi B.. BGP-15, a nicotinic amidoxime derivate protecting heart from ischemia reperfusion injury through modulation of poly(ADP-ribose) polymerase. Biochem Pharmacol 2000;59:937–945. [DOI] [PubMed] [Google Scholar]
- Toner JP, Flood JT.. Fertility after the age of 40. Obstet Gynecol Clin North Am 1993;20:261–272. [PubMed] [Google Scholar]
- Touati SA, Wassmann K.. How oocytes try to get it right: spindle checkpoint control in meiosis. Chromosoma 2016;125:321–335. [DOI] [PubMed] [Google Scholar]
- Wang YA, Farquhar C, Sullivan EA.. Donor age is a major determinant of success of oocyte donation/recipient programme. Hum Reprod 2012;27:118–125. [DOI] [PubMed] [Google Scholar]
- Ward MS, Flemming NB, Gallo LA, Fotheringham AK, McCarthy DA, Zhuang A, Tang PH, Borg DJ, Shaw H, Harvie B. et al. Targeted mitochondrial therapy using MitoQ shows equivalent renoprotection to angiotensin converting enzyme inhibition but no combined synergy in diabetes. Sci Rep 2017;7:15190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LL, Russell DL, Wong SL, Chen M, Tsai TS, St John JC, Norman RJ, Febbraio MA, Carroll J, Robker RL.. Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development 2015;142:681–691. [DOI] [PubMed] [Google Scholar]
- Young ML, Franklin JL.. The mitochondria-targeted antioxidant MitoQ inhibits memory loss, neuropathology, and extends lifespan in aged 3xTg-AD mice. Mol Cell Neurosci 2019;101:103409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng HT, Ren Z, Yeung WS, Shu YM, Xu YW, Zhuang GL, Liang XY.. Low mitochondrial DNA and ATP contents contribute to the absence of birefringent spindle imaged with PolScope in in vitro matured human oocytes. Hum Reprod 2007;22:1681–1686. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu XQ, Lu S, Guo YL, Ma X.. Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Res 2006;16:841–850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.