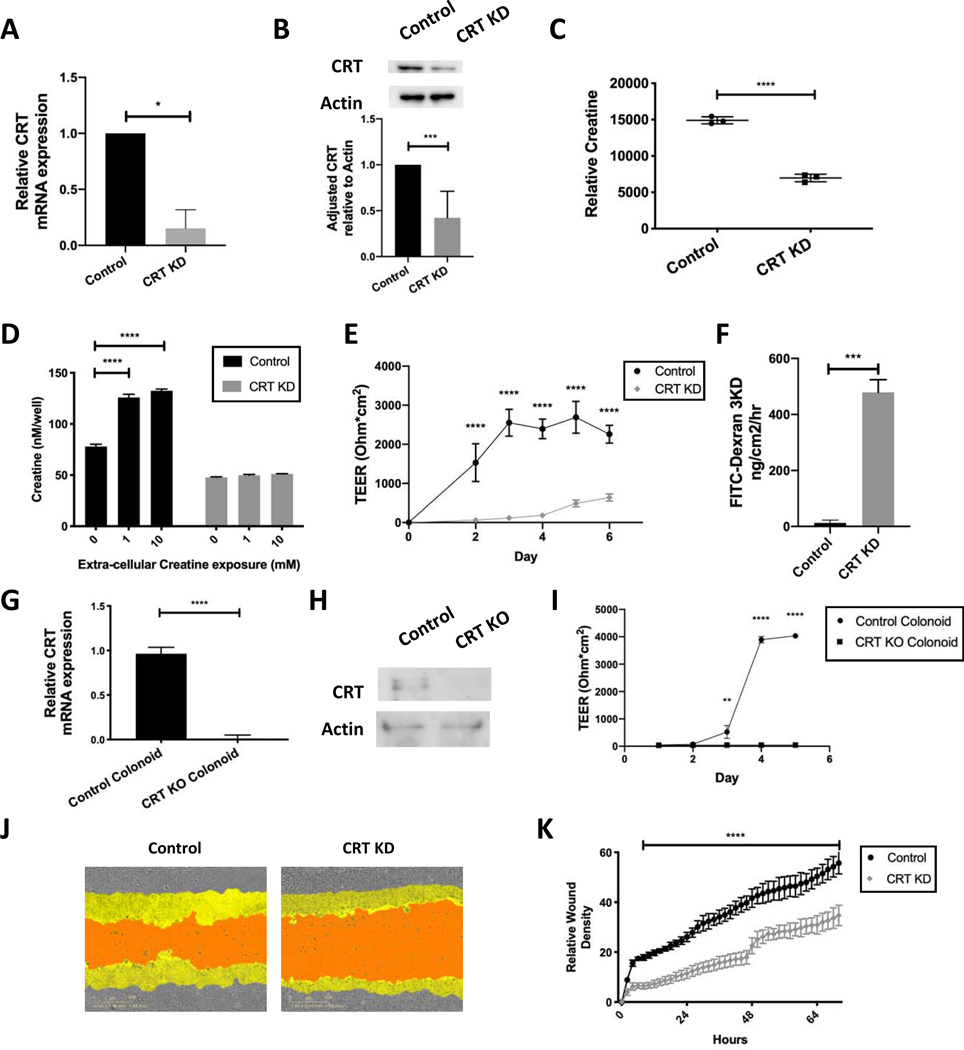
Figure 2. Influence of CRT knockdown on intracellular creatine, barrier function and wound healing in IECs.
A. T84 IECs were transduced with lentiviral shRNA targeting CRT. Cells were selected and mRNA was obtained and analyzed using qPCR. B. Protein was isolated and analyzed by immunoblot(n=5). Densitometry was conducted using ImageJ software. C. CRT KD cell metabolites were isolated and analyzed by HPLC(n=3). D. CRT KD cells were grown in standard growth media with and without creatine supplementation. Cells were washed and intracellular creatine was evaluated using a creatine fluorometric assay(n=3). E. CRT KD cells were grown on a permeable membrane and evaluated for barrier formation by transepithelial electrical resistance (TEER)(n=5). F. CRT KD cells were grown to maximum TEER reading prior to FITC-dextran flux assay(n=3). G. CRT KD cells were grown to confluence and wounded with incucyte wounder. Wound closure was assessed over time by incucyte imager(n=4). H. Relative wound density was calculated Incucyte ZOOM software. I. Colonoids from CRT KO and control mice were evaluated by qPCR for relative CRT expression(n=3). J. Protein was isolated from the mouse CRT KO and control colonoids and analyzed by immunoblot(n=3). K. Barrier analysis by TEER was conducted as previously described using CRT KO and control colonoids(n=3). *p< 0.05, ** p< 0.01, ***p<0.001, ****p<0.0001 determined by unpaired T-test, one-way and two-way ANOVA