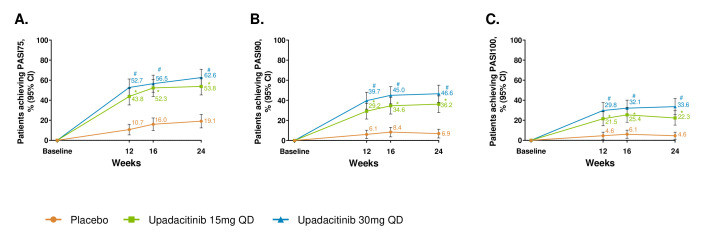
Figure 2.
Proportion of patients achieving (A) PASI75, (B) PASI90 and (C) PASI100. response over 24 weeks. *p≤0.05; for upadacitinib 15 mg QD versus placebo; #p≤0.05; for upadacitinib 30 mg QD versus placebo; †significant in the multiplicity-controlled analysis. After week 16, assessments have been performed. Patients may use concomitant treatments specifically for psoriasis per investigator judgement. Results are based on non-responder imputation. 95% CIs for response rate were calculated based on normal approximation to the binominal distribution. 95% CIs for response rate difference were calculated based on normal approximation. Nominal p value was constructed using Cochran-Mantel-Haenszel test adjusted for the main stratification factor of current disease-modifying antirheumatic drug use (yes/no). PASI75/90/100, 75%/90%/100% improvement in Psoriasis Area Severity Index; QD, once per day.