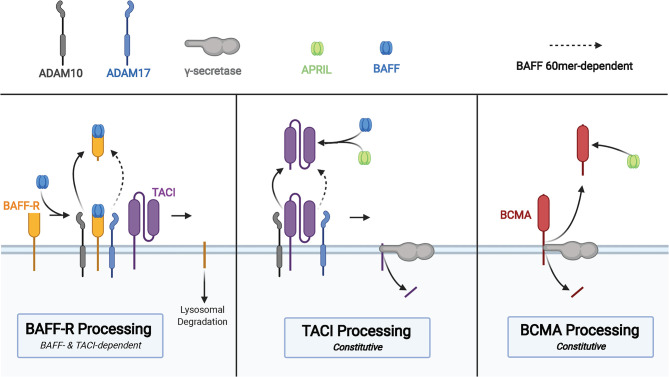
Figure 3.
Membrane processing of human BAFF receptors. Cleavage of the BAFF-R ectodomain is induced by BAFF binding in cells that co-express TACI. Processing of BAFF-R by ADAM10 is induced by binding to BAFF trimers and binding of BAFF 60mer to BAFF-R induces ADAM17 processing of BAFF-R. The membrane-bound C-terminal fragment of BAFF-R is degraded in lysosomes after cleavage of the ectodomain. TACI is cleaved in a constitutive manner by ADAM10, followed by cleavage of the membrane-bound C-terminal fragment by γ-secretase. sTACI exhibits homotypic assembly and binds to BAFF and APRIL to reduce NF-κB activation and B cell survival, with TACI-Fc demonstrating similar capabilities. BCMA is constitutively cleaved by γ-secretase to release sBCMA consisting of the ectodomain and a portion of the transmembrane domain of BCMA. sBCMA is a decoy for APRIL-induced NF-κB activation but does not block BAFF-mediated NF-κB activation. However, BCMA-Fc is capable of binding both APRIL and BAFF to block NF-κB activation.