Figure 5.
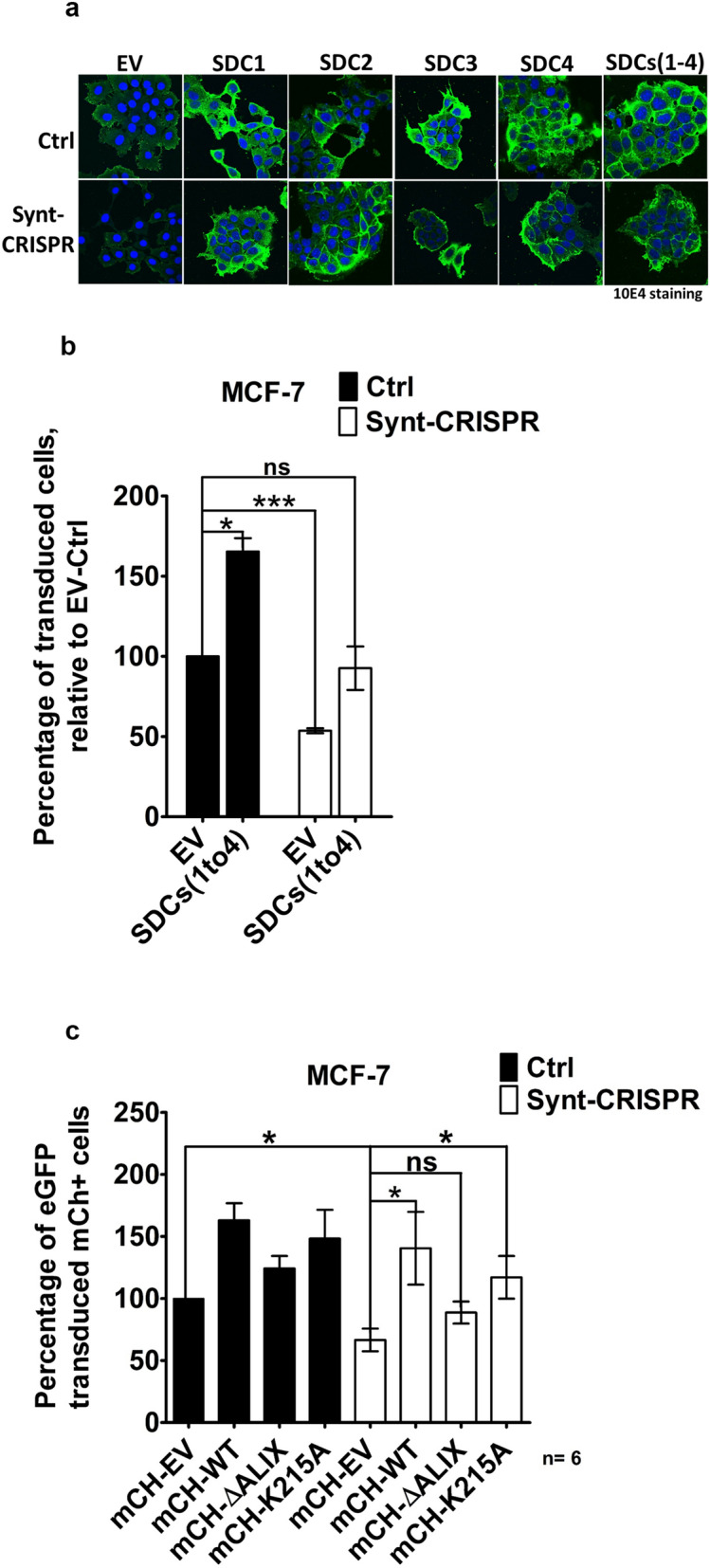
ALIX-binding syntenin rescues retroviral transduction in syntenin 1-negative MCF-7 cells. (a) Representative confocal micrographs of MCF-7 cells showing the distribution of heparan sulfate (as detected by mAb 10E4, green) and the DAPI (blue) staining of the nuclei, upon syndecan over expression (syndecans 1–4, individually and all four together in co-transfection) in control (Ctrl) and in syntenin 1-negative (Synt-CRISPR) MCF-7 cells. (b) Retroviral transfection was analyzed using flow cytometry. Retrovirus encoding LUC IRES eGFP, produced using phoenix packaging cells, was incubated for 48 h with Ctrl and with Synt-CRISPR MCF-7 cells, all or not over-expressing syndecans. Cells expressing eGFP were quantified by flow cytometry. (c) Wild-type MCF-7 cells and Synt-CRISPR MCF-7 cells were transfected with expression plasmid vector encoding mCherry (empty vector), mCherry-syntenin (wild-type syntenin), mCherry-syntenin ∆ALIX (syntenin defective in ALIX-binding) or mCherry-syntenin K215A (syntenin defective in cargo recycling), replated and then incubated for 48 h with retrovirus encoding LUC IRES eGFP. Fluorescent protein expressions were quantified by flow cytometry. eGFP expression in mCherry-expressing cells was taken as a measure of syntenin effects on retroviral transduction. In every experiment, the percentage of wild type cells transfected with empty vector and expressing mCherry that were expressing eGFP was taken as 100 percent. n = 6, bars represent mean values ± SD; n.s., non-significant, *P < 0.05, **P < 0.01, ***P < 0.001 (Student’s t test).
