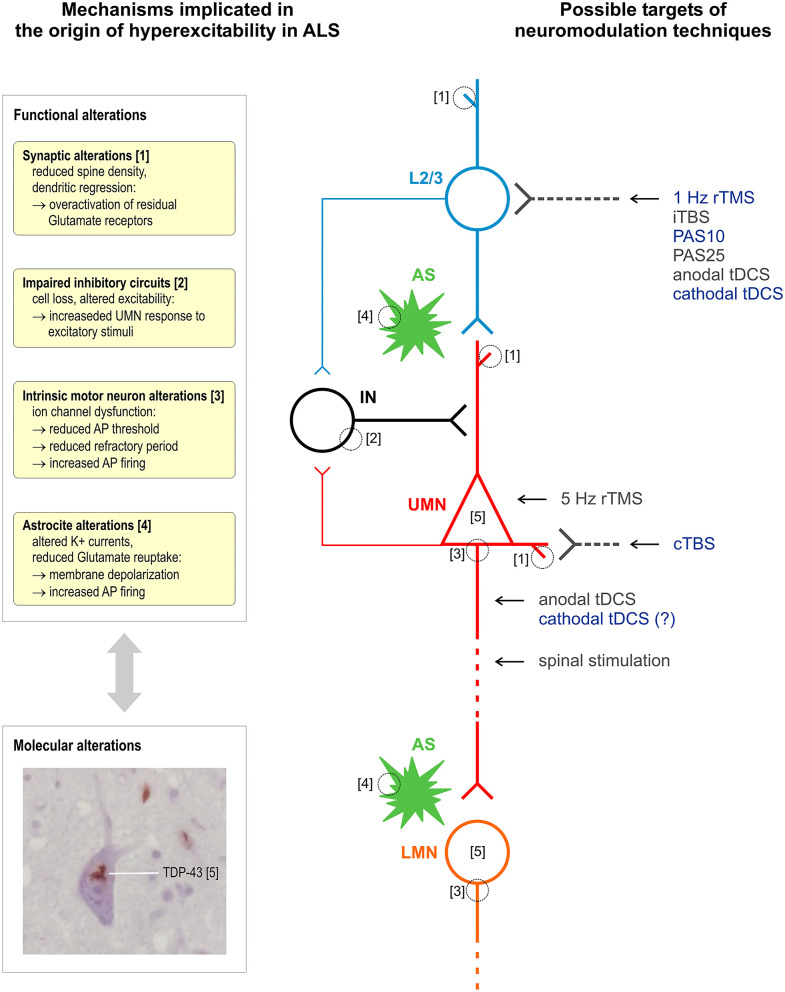
Figure 1.
Schematic representation of mechanisms implicated in the origin of hyperexcitability in ALS and of proposed sites of interaction of different techniques of stimulation of the corticospinal system. Excitatory glutamatergic input to upper motor neurons (UMN, red) is mainly provided by upstream layer 2 and 3 pyramidal neurons (L2/3, light blue) and modulated by astrocytes (AS, green). Different populations of interneurons (IN, black) provide GABAergic input within M1 (a single simplified connection to UMN apical dendrite is represented). All the above cell groups and synaptic connections can be affected by ALS pathophysiological alterations, and current evidence suggests an interplay between functional and molecular alterations, such as TDP-43 cytoplasmic accumulation. It is proposed that most NIBS protocols selectively modulate bursting cells of layers 2 and 3 that project upon layer 5 pyramidal cells or a reverberating local circuit within M1 including GABAergic interneurons; cTBS selectively suppresses the excitability of monosynaptic connections to CSNs; 5-Hz rTMS may produce its effects by enhancing the excitability of CSNs; anodal tDCS and other invasive and non-invasive spinal stimulation techniques interact with corticospinal axons. Stimulation protocols with a documented inhibitory effect on corticospinal excitability are indicated in blue. Histological insert shows TDP-43 skein-like inclusions in the cytoplasm of motor neurons of the hypoglossal nuclei in the medulla oblongata (×100 original magnification). Neuromodulation techniques—rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation; iTBS/cTBS, intermittent/continuous theta burst stimulation; PAS, paired associative stimulation.