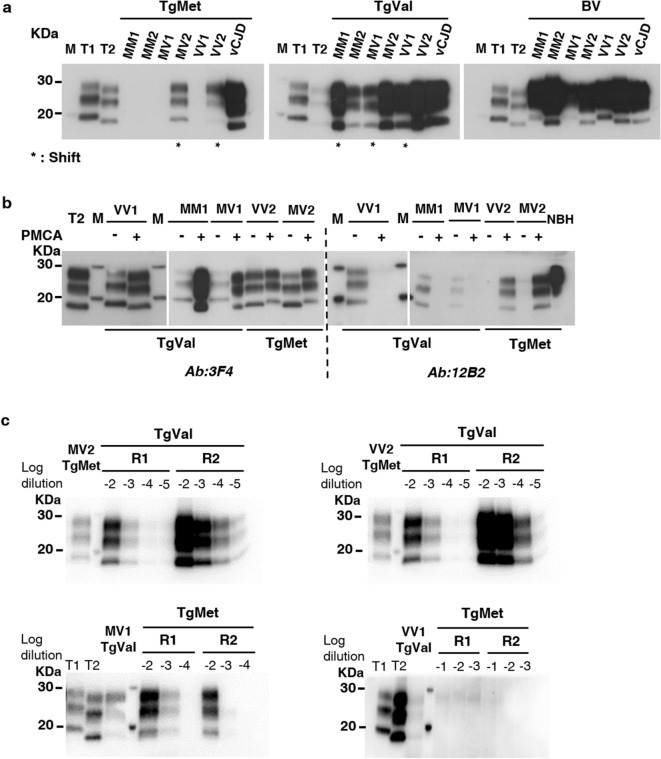
Figure 3.
PrPres shift after PMCA of sCJD according to the substrate. sCJD subtypes as well as vCJD prions were amplified during 4 rounds of PMCA with TgMet, TgVal and BV substrates. The PrPTSE signal was assessed by western blot analysis after proteinase K digestion. T1 corresponds to non-amplified type 1 PrPres (21 kDa) from a 10−3 dilution of either sCJD MM1 or MV1 IBH. T2 corresponds to non-amplified type 2 PrPres (19 kDa) from a 10−3 dilution of either sCJD VV2 or MV2 IBH. M indicates the typical molecular mass of PrPres in the range of 20–30 kDa; (a) PrPres molecular profile after PMCA of sCJD in the different substrates. * indicates generated amplicons with a molecular type1/type2 PrPres shift compared to the initial seed; antibody 9A2; (b) PrPres electrophoretic profiles of shifting PMCA amplicons (+) and their corresponding initial seeds (−) were compared using 3F4 Ab (recognizing both type1 and type2 PrPres) and 12B2 Ab (specific for type1 PrPres). NBH refers to normal brain homogenate from TgMet mice without any proteinase K digestion. For (a) and (b), loaded materials are from different seeded dilutions and have been adjusted in order to better visualize unglycosylated bands: in TgMet: 10−4 for MM1, MM2, MV1 and VV1; 10−5 for MV2 and VV2, 10−8 for vCJD; in TgVal: 10−4 for MM1, MM2, and VV1, 10−5 for MV1 and vCJD, 10−7 for MV2 and VV2; in BV: 10−4 for VV2, 10−5 for MV1, 10−6 for MM1 and VV1, 10−7 for MM2, MV2 and vCJD. (c) Back-seeding of MV2/VV2 amplified in TgMet using TgVal substrate and MV1/VV1 amplified in TgVal using TgMet substrate. MV2 and VV2 PMCA amplicons generated in TgMet substrate (lane 1) were serially diluted from 10−2 to 10−5 and amplified back in TgVal substrate for 2 rounds (R1 and R2). Similarly, MV1 and VV1 PMCA amplicons generated in TgVal substrate (lane 3) were serially diluted from 10−2 to 10−4 for MV1 or from 10−1 to 10−3 for VV1 and amplified back in TgMet substrate for 2 rounds (R1 and R2); antibody: 3F4.