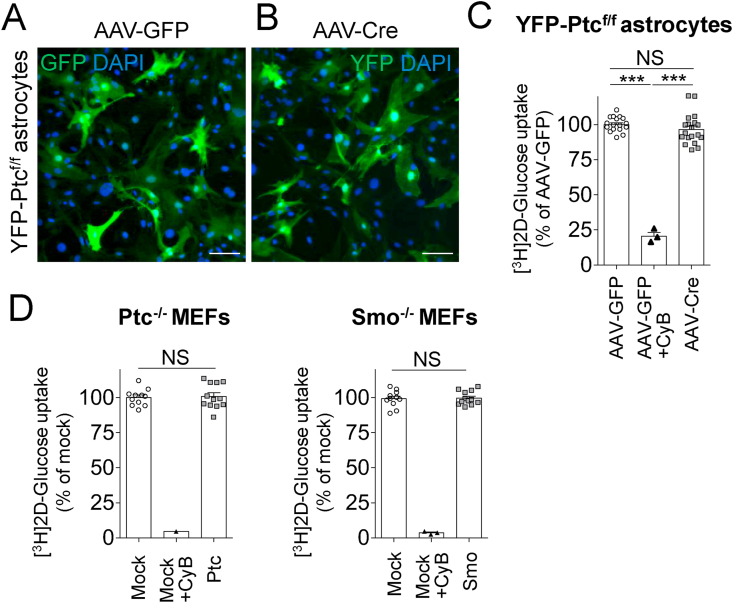
Figure 7.
Glucose uptake was not affected in hypothalamic astrocytes and mouse embryonic fibroblasts upon Ptc deletion in vitro. (A–B) Primary cultures of hypothalamic astrocytes from the YFP-Ptcf/f mice were infected with AAV-GFP (A) as a control or AAV-Cre (B) to induce YFP expression and Ptc deletion upon recombination (see Fig. Sup. 7). Five days post-infection, cells expressed GFP or YFP as detected by immunofluorescence with a GFP antibody. (C) Cytochalasin B (10 μM) inhibited (79.5%) glucose uptake in cultured astrocytes infected with AAV-GFP as assessed using a 15 min [3H]-2-deoxy-d-glucose (3H-DG) uptake assay. No difference in 3H-DG glucose uptake was observed in astrocytes infected with AAV-Cre compared to astrocytes infected with AAV-GFP, indicating that Ptc deletion did not significantly affect the endogenous tone of glucose uptake in these cells. Data are from three infections on three different cultures (n = 17, AAV-GFP; n = 19, AAV-Cre; n = 3, cytochalasin B). (D) Ptc−/- and Smo−/- MEFs were subjected to 3H-DG glucose uptake after transfection with an empty control vector (Mock) or a vector for Ptc (Ptc−/- MEF) or Smo (Smo−/- MEF) expression, respectively, for rescue (see Figure Sup. 7C-D). Analysis of 3H-DG uptake assay (15 min) measured 48 h after transfection indicated no difference between mocked or rescued cells (n = 10–12), whereas cytochalasin B (10 μM) inhibited more than 95% of 3H-DG uptake in mocked cells (n = 3). These data suggested that both Ptc and Smo did not modify the baseline of glucose uptake in these cells. Scale bars, 100 μm. Bar graphs represent mean ± SEM. ∗∗∗p < 0.001 by Student's t test. NS, no significant change.