Abstract
BACKGROUND:
Accumulating evidence suggests that the immune system may be an important target for new treatment approaches in schizophrenia. Positron emission tomography and radioligands binding to the translocator protein (TSPO), which is expressed in glial cells in the brain including immune cells, represents a potential method for patient stratification and treatment monitoring. This study examined whether patients with first-episode psychosis and schizophrenia had altered TSPO levels compared with healthy control subjects.
METHODS:
PubMed was searched for studies comparing patients with psychosis with healthy control subjects using second-generation TSPO radioligands. The outcome measure was total distribution volume (VT), an index of TSPO levels, in frontal cortex, temporal cortex, and hippocampus. Bayes factors (BFs) were applied to examine the relative support for higher, lower, or no difference in patients’ TSPO levels compared with healthy control subjects.
RESULTS:
Five studies, with 75 participants with first-episode psychosis or schizophrenia and 77 healthy control subjects, were included. BFs showed strong support for lower VT in patients relative to no difference (all BFs > 32), or relative to higher VT (all BFs > 422), in all brain regions. From the posterior distributions, mean patient–control differences in standardized VT values were −0.48 for frontal cortex (95% credible interval [CredInt] = −0.88 to 0.09), −0.47 for temporal cortex (CredInt = −0.87 to −0.07), and −0.63 for hippocampus (CredInt = −1.00 to −0.25).
CONCLUSIONS:
The lower levels of TSPO observed in patients may correspond to altered function or lower density of brain immune cells. Future studies should focus on investigating the underlying biological mechanisms and their relevance for treatment.
Keywords: Immune activation, Meta-analysis, Microglia, Positron emission tomography, Psychosis, Schizophrenia, Translocator protein
Genetic, epidemiological, and biomolecular data suggest that the immune system is involved in the pathophysiology of schizophrenia (1–3). When translating these findings into clinical trials, initial studies have shown a positive effect of medication targeting the immune system when used as an add-on treatment to antipsychotics (4–6). To aid further development of this therapeutic approach, tools for directly assessing the status of the brain immune system are needed to allow for patient stratification and monitoring of treatment effects.
Using positron emission tomography (PET), the localization and activation state of central nervous system immune response modulators can be assessed with radioligands targeting the 18-kDa translocator protein (TSPO), which is expressed in glial cells (7–9). During the last decade, a handful of TSPO PET studies have been performed in patients with early-stage psychosis or manifest schizophrenia, showing inconclusive results. Early reports using the first-generation TSPO radioligand (R)-[11C]PK11195 showed higher binding in small patient groups (n = 7 and n = 10) (10,11), albeit with outcome measures that show low accuracy and reliability (i.e., binding potential estimated from rate constants) (12–14). More recent studies in larger samples using the same radioligand, but without blood sampling for full quantification, did not replicate these findings (15–17). Concerns regarding the low signal-to-noise ratio of (R)-[ 11C]PK11195 sparked the development of a series of second-generation TSPO radioligands, showing much greater specific binding (18–21). These tools have subsequently been used to revisit the question of higher levels of TSPO in psychosis (22–26). When employing gold standard outcome measures of binding in the absence of a reference region (total distribution volume [VT] obtained using kinetic modeling with metabolite-corrected arterial plasma as input function), higher TSPO expression has so far not been found in patients. In some cases, trend-level (24) or significantly lower (23) TSPO levels were shown.
All previous TSPO PET studies in psychosis have been performed with relatively small sample sizes. In addition, TSPO radioligands display substantial within- and between-subject variability (12,27) even after accounting for the TSPO rs6971 polymorphism that is known to affect radioligand binding in vivo (28–30). This has important implications for sensitivity and the power to detect differences between patients with psychosis and control subjects. Indeed, the power to detect an expected significant medium-sized difference between diagnostic groups (at alpha = .05) has ranged from 23% to 34% in previous designs (22–26). Medication status has also differed both between and within these studies. Because antipsychotics have been shown to dampen the immune response, this further limits the conclusions that can be drawn (31). Here, we sought to overcome these limitations and clarify the use of TSPO PET as a biomarker of immune dysfunction in schizophrenia. We conducted an individual participant data (IPD) meta-analysis of all TSPO PET studies performed in psychosis or schizophrenia using second-generation radioligands, where VT was included as the outcome measure. The primary objective was to evaluate the hypotheses of 1) higher, 2) lower, or 3) no difference in VT between patients and healthy control subjects (HCs). A secondary objective was to assess the effects of antipsychotic medication on TSPO levels.
METHODS AND MATERIALS
Preferred Reporting Items for Systematic Reviews and Meta-analyses, Preregistration, and Code Availability
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses of IPD (PRISMA-IPD) (32) and according to a study-specific preregistration protocol. The preregistration protocol and all codes used in this study can be found on the public repository (https://github.com/pontusps/TSPO_psychosis).
Selection Criteria and Search Strategy
We set out to obtain IPD from all PET studies that 1) used a second-generation TSPO radioligand, 2) reported VT values in the central nervous system in subjects with psychosis or schizophrenia as compared with HCs, and 3) reported TSPO affinity type of all participants. To our knowledge, there are currently five published studies reporting such data, using the radioligands [11C]PBR28, [18F]FEPPA, and [11C]DPA713 (22–26). To ascertain that no relevant studies were omitted from this meta-analysis, we performed a systematic literature search on PubMed. Only articles published after 2004 were included in the search, corresponding to the year when the first report on a second-generation TSPO radioligand was published (33). Search terms included (among others) “psychotic disorder,” “schizophrenia,” “positron emission tomography,” “translocator protein 18 kDa,” and “peripheral benzodiazepine receptor” (for the full list of search terms, see the Supplement). All TSPO PET studies in psychosis or schizophrenia that were not included are listed in Supplemental Table S1 along with a detailed explanation of the selection criteria. Corresponding authors of eligible studies were contacted via e-mail, and all agreed to contribute.
Requested Data
Requested IPD included VT values from the frontal cortex (FC), temporal cortex (TC), and hippocampus (HIP) regions of interest (ROIs), patient–control status, TSPO genotype, age, gender and medication status, Positive and Negative Syndrome Scale (PANSS) scores (or equivalent), and duration of illness. These three ROIs were selected because four of five included studies had reported VT values from all of them. For the remaining study [Bloomfield et al. (22)], unpublished IPD VT values obtained using the conventional two-tissue compartment model from all three ROIs were provided on request, allowing for consistent pooling. To account for range differences among different radioligands used across studies, we Z-scored all ROI VT values within each genotype group of each study.
Quality Control
The first author (PP-S) examined the integrity of the obtained IPD datasets. The data were checked for outliers and inconsistencies with the published data (such as number of participants, means, ranges, and SDs of VT and age), which were then resolved following discussion with the authors of the relevant studies.
Meta-analysis and Statistics
The studies included in this meta-analysis recruited participants of two different TSPO affinity types (high-affinity binders and mixed-affinity binders), used different radioligands, and applied different image analysis procedures. To estimate the difference in VT between diagnostic groups (ΔVT) while taking this hierarchical structure into account, we constructed and compared four different Bayesian linear mixed-effects (BLME) models of increasing complexity: In model 1 (M1), standardized ROI VT was specified as the dependent variable, diagnostic group as the fixed effect, and genotype and study as random effects with varying intercepts. Model 2 (M2) was the same as M1 but with varying slopes of the random effect of genotype (i.e., allowing for differences in ΔVT between high-affinity binders and mixed-affinity binders). Model 3 (M3) was the same as M1 but with varying slopes of the random effect of study (i.e., allowing for differences in ΔVT between studies). Model 4 (M4) was the same as M1 but with varying slopes for both random effects (i.e., allowing for differences in ΔVT between genotypes and studies). The model with the best fit to data, as determined by widely applicable information criterion and leave-one-out cross-validation scores, was selected (34).
Following model selection, we first examined the hypothesis that patients with psychosis or schizophrenia have higher levels of TSPO in the brain (hypothesis 1 [H1]). For each ROI, we quantified the relative evidence of higher TSPO expression in patients compared with the null hypothesis of no difference (H0). This was done using order-restricted Bayes factor (BF) hypothesis testing (35–37) on ΔVT. BF quantifies the relative evidence, or support, for one hypothesis over another as a ratio of their average likelihoods. A BF > 10 is usually considered as strong evidence in favor of a hypothesis (and, consequently, BF < 0.1 translates into strong evidence of the opposite hypothesis) (35). We calculated BFH1:H0 to quantify the evidence in favor of higher ROI VT in patients compared with control subjects relative to no difference. Second, we examined whether patients had lower levels of VT in the ROI (H2). Again, this was done by employing an order-restricted BF test of lower VT in patients (BFH2:H0) over no difference. Finally, we calculated the support for H2 over H1 (BFH2:H1), signaling the relative likelihood of lower levels of TSPO in patients compared with higher levels.
For each ROI, H1 and H2 were specified as half-Gaussian (normal) distributions centered on zero with a standard deviation of 0.5. Hence, to perform order-restricted hypothesis testing of patient–control differences, the priors over ROI ΔVT were specified as half Gaussians (SD = 0.5) with a lower bound of zero for H1 and an upper bound of zero for H2. The Savage–Dickey ratio method was then used to calculate BFs. The standard deviation was set a priori to 0.5 because this assigns high plausibility to ΔVT values ranging from 0 to a medium-sized difference (38,39). A medium-sized difference, corresponding to a Cohen’s d of 0.5, was considered a reasonable prediction based on the precision of the outcome measure (27). A medium effect size (Cohen’s d = 0.5) group difference in VT means that 69% of the patient population would be expected to have a higher (or lower) VT than the mean of the population of HCs [Cohen’s U3 (38)].
A robustness check of the effect of different prior widths on BF was performed by varying the SDs of the half-Gaussian distributions (SDs = 0.2 and 0.8, corresponding to expected small and large effect sizes of ΔVT and Cohen’s U3 = 58% and 79%, respectively) when testing all hypotheses. For the prior on the SDs of the random effects, half-Cauchy distributions (with a scale of 0.707) were used. These weakly informative priors were chosen because the numbers of genotype groups (n = 2) and studies (n = 5) are small (40).
We also estimated the overall effect size of standardized VT difference between patients and HCs. This was done using M3 with a nontruncated, weakly regularizing prior (Gaussian with an SD of 10) over the fixed effect. M3 was selected because it also allowed us to extract the study-specific effects of ROI ΔVT (random slopes) and the corresponding SDs of these effects (τ). Using these, we produced a forest plot of ROI ΔVT and examined τ as a measure of study heterogeneity, in line with the PRISMA-IPD guidelines.
For the secondary aim of analyzing medication effects on VT, we added an additional predictor, denoting medication status, to the best-fitting BLME model. This predictor quantifies the additional effect of being medicated after controlling for patient–control status. For each ROI, the prior distribution over the beta coefficient was a nontruncated Gaussian centered on zero with an SD of 10. The posterior of this predictor was then extracted together with its summary statistics (mean and 95% credible interval [CredInt]) to examine the effect of medication.
We also examined the correlation between ROI VT values and PANSS-Positive scores, PANSS-Negative scores, and duration of illness using linear effect modeling, allowing the correlations to vary between studies. All data were Z-transformed within study (and within genotype for VT).
The primary reason for choosing Bayesian statistical inference is that the BF allows for a direct comparison of the evidence for one hypothesis relative to another hypothesis (such as H1 against H2, i.e., higher TSPO in patients vs. lower TSPO in patients). Bayesian parameter estimation also allowed us to assess and report the uncertainty around parameters in the model, which guards against overconfidence and overfitting when making inference. For completeness, we also present frequentist equivalents of the best-fitting model, showing p values for patient–control differences in standardized VT for each ROI in Supplemental Table S2. The Hamiltonian Markov chain Monte Carlo sampler Stan (41) and the R packages brms (42) and lme4 (43) were used for the statistical modeling in this meta-analysis.
RESULTS
Study Selection and Data Collection
The PubMed search was performed on February 20, 2017, and resulted in 13 research articles. The articles were read in full by two of the authors (PP-S and SC). Both authors concluded independently that five studies (22–26) fulfilled the inclusion criteria for this meta-analysis (see PRISMA flowchart in Supplemental Figure S3). Each corresponding author provided anonymized individual participant VT values from FC [three studies (22–24)], dorsolateral prefrontal cortex [two studies (25,26)], TC (all studies), and HIP (all studies). For all subsequent analyses in this study, the VT values from FC and dorsolateral prefrontal cortex were considered to represent the same ROI.
Characteristics of Included Studies
Table 1 shows demographic information, medication status, PANSS (or equivalent), and duration of illness of all participants included in this meta-analysis. In total, IPD from 75 participants with psychosis or schizophrenia and 77 HCs were included in the statistical analysis. All patients who participated in Kenk et al. (26) and Bloomfield et al. (22), and all patients except 2 who participated in Coughlin et al. (24), were on antipsychotic treatment at the time of PET. Of the 19 patients who participated in Hafizi et al. (25), 5 were antipsychotic free with less than 4 weeks of lifetime cumulative exposure and 14 were antipsychotic naive at the time of scanning. All patients in Collste et al. (23) were antipsychotic naive. For all studies, exclusion criteria included clinically significant medical comorbidity and substance abuse. In two of the studies benzodiazepines were not allowed (22,24), whereas in Collste et al. (23) and Kenk et al. (26) the results did not change when removing subjects using benzodiazepines. Based on this information, as well as in vitro data showing effects of only high doses of diazepam on TSPO levels (44), we chose not to include this variable in our analysis. For information on recruitment of HCs, quality control of the data, and assignment of subjects who overlapped in the original studies, see the Supplement. Figure 1 displays the individual participant ROI VT values from the five studies included in this meta-analysis.
Table 1. Descriptive Characteristics of Included Data.
| Study | Diagnostic Group | Schizophrenia/Other | Age, Mean (SD), Years | Count | HABs | MABs | Men | Women | PANSS-T, Mean (SD) | PANSS-P, Mean (SD) | PANSS-N, Mean (SD) | DOI Mean (SD), Months | Drug Freea/Total | Radioligand | Original Result |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bloomfield et al. (22) | HCs | – | 46.21 (13.62) | 14 | 14 | 0 | 11 | 3 | – | – | – | – | – | [11C]PBR28 | N.S. |
| Pat | 12/0 | 47.00 (9.31) | 12 | 12 | 0b | 9 | 3 | 63.7 (18.1) | 17.0 (6.1) | 14.1 (4.0) | 108.9 (46.7) | 0/12 | |||
| Collste et al. (23) | HCs | – | 26.38 (8.44) | 16 | 9 | 7 | 7 | 9 | – | – | – | – | – | [11C]PBR28 | ↓Pat |
| Pat | 4/12d | 28.50 (8.37) | 16 | 8 | 8 | 11 | 5 | 77.4 (18.3) | 20.3 (4.9) | 18.1 (7.0) | 7.9 (9.6) | 16/16 | |||
| Coughlin et al. (24) | HCs | – | 25.36 (4.89) | 14 | 9 | 5 | 9 | 5 | – | – | – | – | – | [11C]DPA173 | N.S. |
| Pat | 12/0 | 24.33 (3.28) | 12 | 8 | 4 | 9 | 3 | – | 13.8 (2.7)c | 15.8 (4.6)c | 25.0 (16.3) | 2/12 | |||
| Hafizi et al. (25)e | HCs | – | 27.17 (9.07) | 18 | 14 | 4 | 8 | 10 | – | – | – | – | – | [18F]FEPPA | N.S. |
| Pat | 15/4f | 27.53 (6.78) | 19 | 14 | 5 | 12 | 7 | 68.6 (13.0) | 19.2 (3.8) | 16.1 (6.1) | 33.6 (40.1) | 19/19 | |||
| Kenk et al. (26)e | HCs | – | 54.27 (9.51) | 15 | 10 | 5 | 7 | 8 | – | – | – | – | – | [18F]FEPPA | N.S. |
| Pat | 16/0 | 42.50 (14.03) | 16 | 10 | 6 | 10 | 6 | 70.2 (9.7) | 19.3 (2.2) | 18.6 (5.0) | 177.3 (105.7) | 0/16 | |||
| All | HCs | – | 35.42 (15.12) | 77 | 56 | 21 | 42 | 35 | – | – | – | – | – | – | – |
| Pat | 59/16 | 33.88 (12.57) | 75 | 52 | 23 | 51 | 24 | – | 18.2 (4.2) | 16.6 (5.5) | 72.1 (57.2) | 37/77 | |||
DOI, duration of illness; HABs, high-affinity binders; HCs, healthy control subjects; MABs, medium-affinity binders; N.S., nonsignificant; PANSS-N, PANSS–Negative score; PANSS-P, PANSS–Positive score; PANSS-T, Positive and Negative Syndrome Scale–Total score; Pat, participants with psychosis or schizophrenia; ↓Pat, lower total distribution volume in subjects with psychosis.
Drug-naive patients (n = 30) or patients not medicated with antipsychotics at the time of the positron emission tomography examinations (n = 7).
The 2 MAB subjects from the patient group were excluded from the hierarchal inferential analyses because Z-scoring within genotype was not meaningful.
PANSS-P score converted from Scale for the Assessment of Positive Symptoms score, and PANSS-N score converted from Scale for the Assessment of Negative Symptoms score, using van Erp et al. (74).
Other diagnoses: 7 schizophreniform disorder, 4 psychosis not otherwise specified, and 1 brief psychosis.
The 14 HCs shared across the Kenk et al. (26) and Hafizi et al. (25) studies have been uniquely assigned to either one of the studies. Assignment was done as to best match the patient groups based on count, genotype, gender, and age.
Other diagnoses: 3 schizophreniform and 1 delusional disorder.
Figure 1.
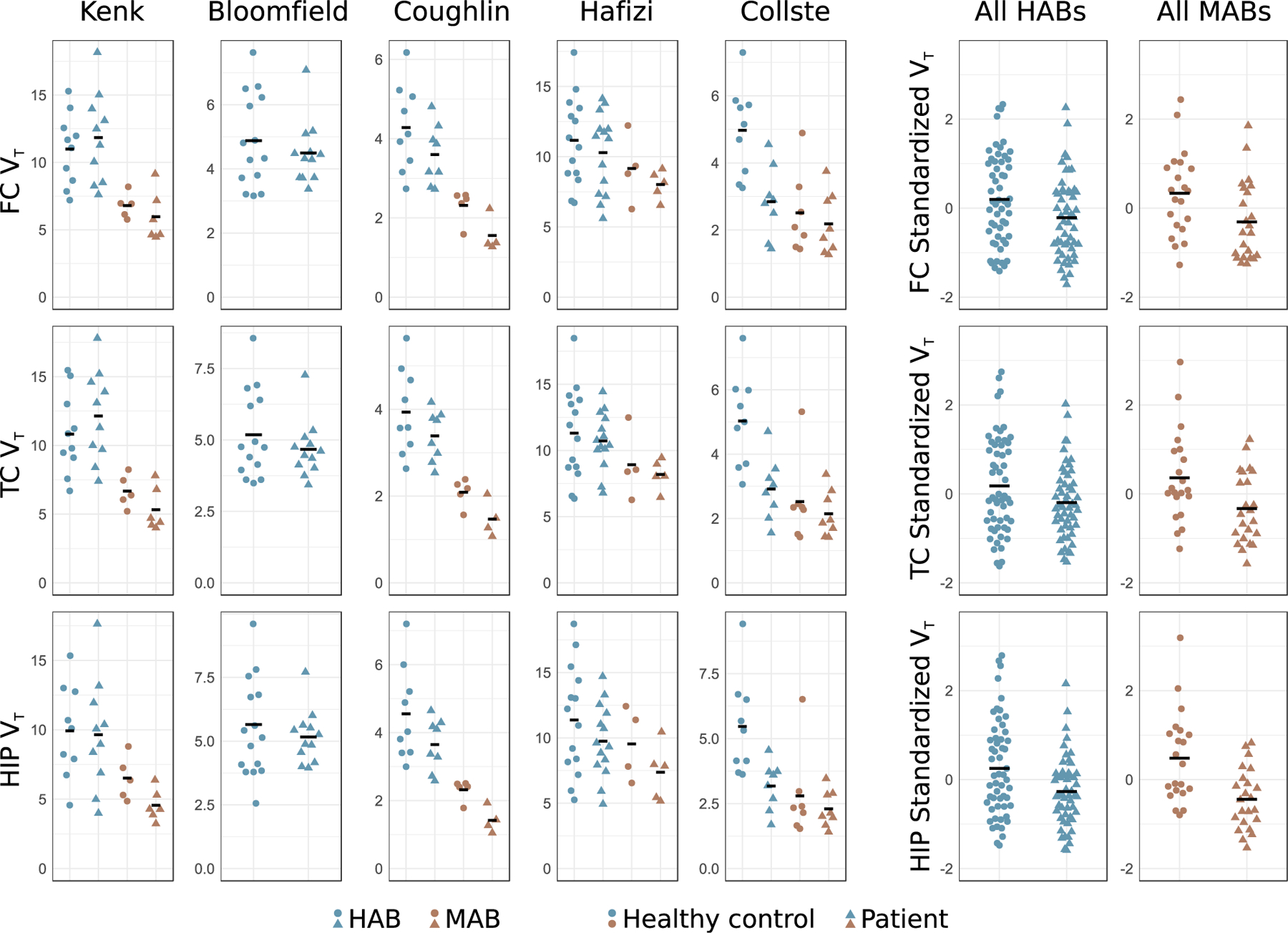
Individual participant raw data showing translocator protein levels (estimated using total distribution volume [VT]) in participants with first-episode psychosis or schizophrenia and healthy control subjects, from all five included studies, from frontal cortex (FC), temporal cortex (TC), and hippocampus (HIP). The black bars denote the group means. For each region, subjects’ VT values have been Z-scored within study, and within genotype, in order to produce the pooled plots of all high-affinity binders (HABs) and mixed-affinity binders (MABs). For this reason, HABs and MABs have the same mean (set to zero) in the right-hand panels. Included studies: Bloomfield et al. (22); Collste et al. (23); Coughlin et al. (24); Hafizi et al. (25); Kenk et al. (26).
The mean age of all subjects in the patient group was 33.88 years (SD = 12.57), and the mean age of all subjects in the HC group was 35.42 years (SD = 15.12). This corresponds to a negligible difference in age between diagnostic groups (Cohen’s d = 0.11). Fisher’s exact test indicated some skewness in gender distribution between the patient and control groups (p = .0504). To ascertain that any potential differences in ROI VT values between diagnostic groups in the main analysis were not driven by gender differences, we included gender as a covariate and executed an additional set of BLME models, using the same procedure as outlined in Methods and Materials. It should be noted that we had no information regarding the menstrual cycle, which could potentially influence the results in female participants, although relationships between TSPO and menstrual cycle hormonal levels have as of yet to be demonstrated.
Model Selection
M1 showed a slightly better fit, determined by widely applicable information criterion and leave-one-out cross-validation scores, compared with M2 and M3 (Table 2). Therefore, we used M1 to obtain order-restricted posterior distributions of ROI ΔVT and subsequently quantified evidence in favor of H0, H1, and H2.
Table 2. Model Fits for Four Different Bayesian Linear Mixed-Effects Models Examining the Difference in TSPO Binding (Estimated Using VT) Between Patients With Psychosis and Healthy Control Subjects.
| Region | Model | dLOOC | dWAIC | Akaike Weighta (%) |
|---|---|---|---|---|
| Frontal Cortex | 0 | 7.6 | 7.6 | 1 |
| 1 | 0 | 0 | 38 | |
| 2 | 0.8 | 0.8 | 26 | |
| 3 | 1.1 | 1.1 | 22 | |
| 4 | 1.9 | 1.9 | 14 | |
| Temporal Cortex | 0 | 7.1 | 7.1 | 1 |
| 1 | 0 | 0 | 35 | |
| 2 | 0.6 | 0.6 | 26 | |
| 3 | 0.9 | 0.9 | 22 | |
| 4 | 1.6 | 1.6 | 16 | |
| Hippocampus | 0 | 15.3 | 15.4 | < 1 |
| 1 | 0 | 0 | 36 | |
| 2 | 0.4 | 0.4 | 29 | |
| 3 | 1.3 | 1.2 | 19 | |
| 4 | 1.7 | 1.6 | 16 |
A null model (0) without patient–control status as predictor is included as a baseline comparison. Lower dLOOC and dWAIC values indicate better model fit.
dLOOC, distance to best-fitting model calculated using leave-one-out cross-validation; dWAIC, distance to best-fitting model calculated using widely applicable information criteria; TSPO, translocator protein; VT, total distribution volume.
Weights calculated using LOOC scores.
Patient–Control Difference in VT (Primary Aim)
BFH1:H0 values in favor of higher VT in patients (H1) were 0.08 for FC, 0.08 for TC, and 0.06 for HIP. This translates into strong support for the null hypothesis of no difference (H0) relative to higher levels of TSPO in patients. BFH2:H0 values in favor of lower VT in patients (H2) were 32.5 for FC, 34.2 for TC, and 1481.0 for HIP compared with H0. This signifies very strong evidence for the hypothesis that patients express lower TSPO levels. As a result, there was extremely strong support for H2 over H1 (BFH2:H1 values: FC = 422.9; TC = 440.6; HIP = 24524.0). Hence, lower VT in patients with psychosis, as compared to with HCs, is more than 422 times more likely than higher VT, conditioned on the data and the models (see Table 3 and Supplemental Figure S1 for all computed BFs).
Table 3. Bayes Factors (BF) of Hypothesis Testing of the Difference in Standardized Brain TSPO Binding (Estimated Using VT) Between Patients and Control Subjects Using the Best-Fitting Model (M1).
| Region | H0:H1 | H1:H0 | H0:H2 | H2:H0 | H1:H2 | H2:H1 |
|---|---|---|---|---|---|---|
| FC | 13.0 | 0.08 | 0.03 | 32.5 | 0.002 | 422.9 |
| TC | 12.9 | 0.08 | 0.03 | 34.2 | 0.002 | 440.6 |
| HIP | 16.6 | 0.06 | 0.001 | 1481.0 | < 0.001 | 24524.0 |
FC, frontal cortex; H0, null hypothesis; H1, hypothesis 1; H2, hypothesis 2; H0:H1, BF denoting evidence in favor of H0 over H1; H1:H0, BF denoting evidence in favor of H1 over H0; H0:H2, BF denoting evidence in favor of H0 over H2; H2:H0, BF denoting evidence in favor of H2 over H0; H1:H2, BF denoting evidence in favor of H1 over H2; H2:H1, BF denoting evidence in favor of H2 over H1; HIP, hippocampus; M1, Model 1; TC, temporal cortex; TSPO, translocator protein; VT, total distribution volume.
When varying the widths (SDs = 0.2 and 0.8) of the Gaussian prior distribution on the fixed effect of differences between patients and control subjects, there was still strong support in favor of H2 for all ROIs (all BFH2:H0 > 15) (see Supplemental Table S3). The addition of gender as a covariate did not change the qualitative inference for any of the ROIs (all BFH2:H0 > 16) (see Supplemental Table S4).
Estimation of Effect Sizes and Study Heterogeneity
For estimation of effect sizes and study heterogeneity, M3, with an uninformative prior over ΔVT, was used. Figure 2 displays forest plots of the estimated patient–control difference in each study for each ROI. It also shows the posterior distributions of the standardized ΔVT across all studies together with summary statistics (mean and credible intervals). The mean of each ROI’s posterior distribution corresponded to a medium-sized (i.e., Cohen’s d ≈ 0.5) difference in VT between patients and control subjects. When calculating group differences using raw VT values, subjects with psychosis or schizophrenia had, on average, 15% lower VT in FC, 14% lower VT in TC, and 24% lower VT in HIP compared with HCs.
Figure 2.
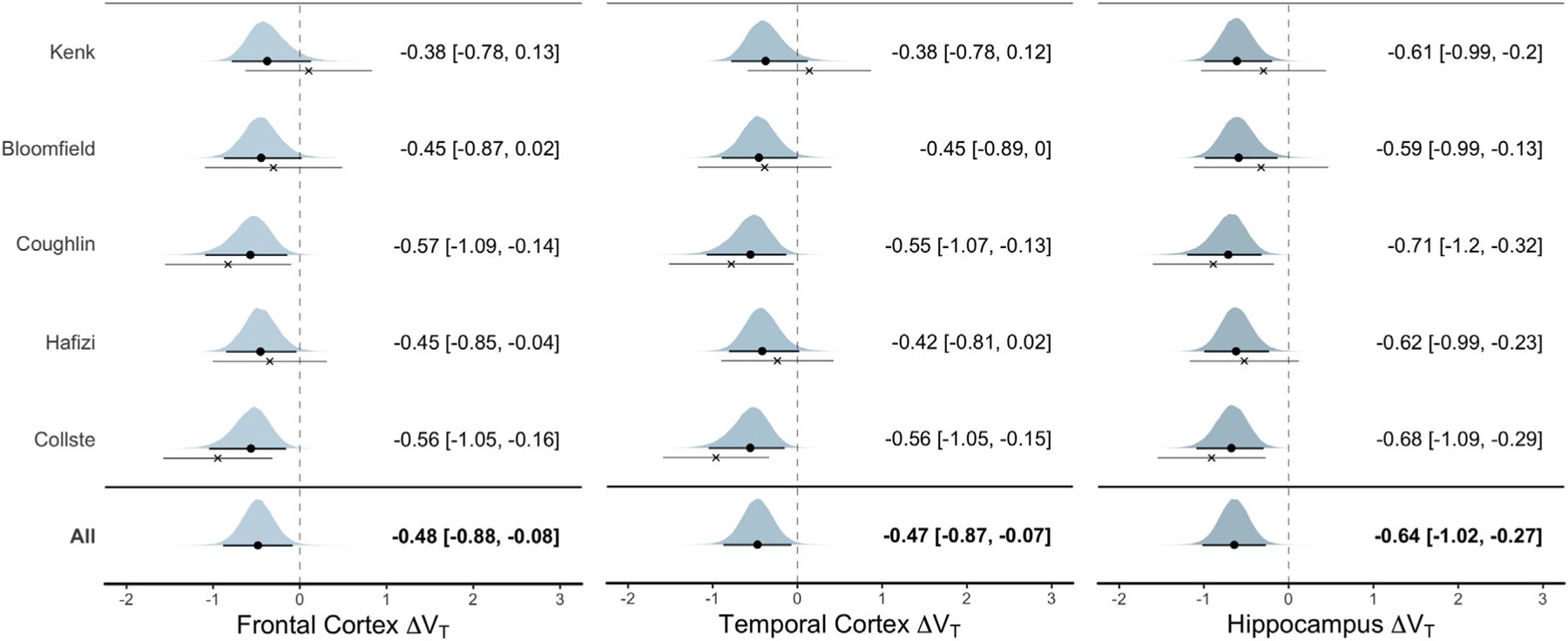
Standardized difference in translocator protein levels (estimated using total distribution volume [VT]) between patients with psychosis and healthy control subjects. The posterior distribution for each study-specific difference in VT (ΔVT) estimate (random slopes) from the linear mixed model is presented. The black circle denotes the posterior mean, and the thick line denotes the 95% credible interval; these are also presented in text next to the plots. The cross denotes the patient–control mean difference in raw data (together with its 95% credible interval) without performing linear mixed-effects modeling. Hence, the difference between the dot and the cross displays the model shrinkage toward the mean. The overall ΔVT estimate suggests that patients with schizophrenia or first-episode psychosis have lower levels of translocator protein compared with healthy control subjects. Included studies: Bloomfield et al. (22); Collste et al. (23); Coughlin et al. (24); Hafizi et al. (25); Kenk et al. (26).
For all ROIs, the SDs of the random slopes of studies (τ) were very small (posterior modes < 0.04; posterior means < 0.22) and I2 < 15%, signifying low study heterogeneity in ΔVT differences (see Supplemental Figure S2).
Effect of Medication (Secondary Aim)
We examined the effect of medication on VT by adding medication status as an additional predictor to M1. For all ROIs, the models showed little to no evidence of a medication effect, allocating as much probability to higher VT as they did to lower VT. The means of the posterior over the difference in standardized VT due to medication were 0.009 for FC (CredInt = −0.384 to 0.401), −0.013 for TC (CredInt = −0.407 to 0.381), and −0.040 for HIP (CredInt = −0.423 to 0.343) (see Figure 3). Thus, no support was found for a difference in TSPO levels between drug-free and medicated patients.
Figure 3.
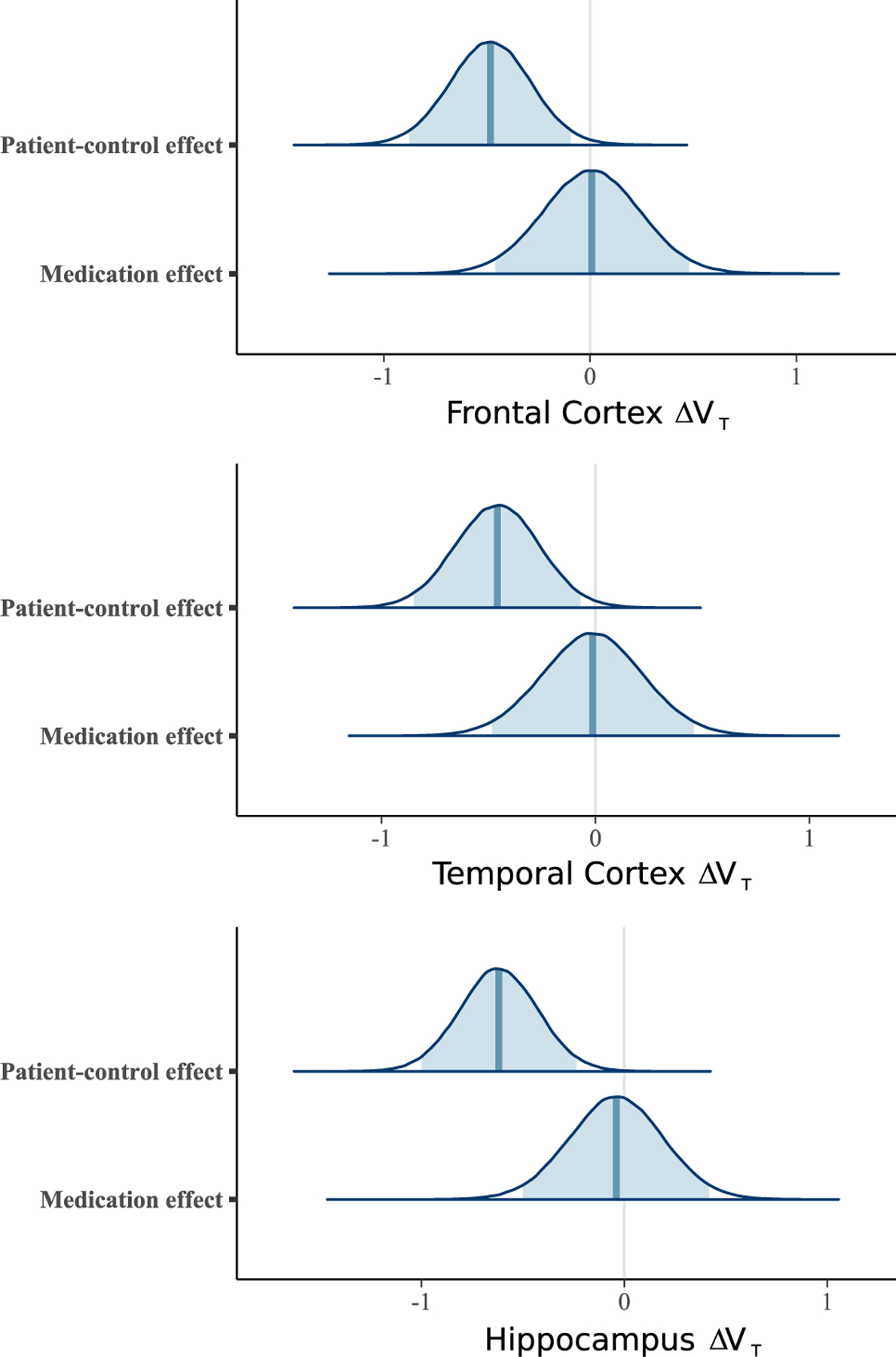
Posterior distributions over the differences in standardized brain translocator protein levels (estimated using total distribution volume [VT]) between patients and control subjects and the additional effect of medication status (being medicated with antipsychotics or not at the time of positron emission tomography). The posterior distributions of medication effect are centered on zero and suggest that antipsychotic treatment does not affect brain VT after accounting for differences between patients with psychosis or schizophrenia and control subjects. ΔVT, difference in VT.
There was little to no evidence for a correlation between regional VT values and PANSS-Positive scores, PANSS-Negative scores, or duration of illness (see Supplemental Figures S5 and S6 and Supplemental Tables S5 and S6).
DISCUSSION
The main finding of this IPD meta-analysis was that patients with schizophrenia and first-episode psychosis showed lower levels of the glial cell marker TSPO compared with HCs. Using BLME modeling, we observed very strong evidence of lower levels of TSPO, measured using VT, in FC, TC, and HIP, contrary to the hypothesis of higher TSPO in patients. As such, this study constitutes the most conclusive in vivo investigation of TSPO in psychosis to date.
Antipsychotic medication has been shown to attenuate blood cytokine levels in patients (31) as well as to inhibit immune cell activity in vitro (45). Although the effect on TSPO expression in animals is less conclusive (46), these observations suggest that TSPO levels could be lower in medicated subjects compared with unmedicated subjects. However, our secondary analysis of the effect of medication status yielded no evidence for such a difference in radioligand binding between drug-free and medicated patients. This indicates that the observed lower levels of TSPO in patients is not an effect of exposure to antipsychotic treatment.
A wealth of data has demonstrated higher levels of proinflammatory markers, such as cytokines, in cerebrospinal fluid and plasma in patients across disease stages of schizophrenia (3,47). In the brain, these signaling molecules are mainly released by microglia and astrocytes, which have key roles in the immune response (9). Therefore, increases in numbers or activity of these cells in schizophrenia have been hypothesized (48,49). In postmortem studies, higher levels of brain glial cell markers, such as human leukocyte antigen–antigen D related and CD11b, have been observed in patients, although results have been mixed (50–52). With regard to astrocyte markers, there is no evidence of any overall differences between patients and control subjects (51,52). In the case of TSPO, which is expressed in microglia and astrocytes among other cells (8,9,53), autoradiographic studies have reported both higher (28) and lower (54) binding in patients as compared with HCs. Important caveats when interpreting these studies are that the age of patients and control subjects is generally high and the cause of death in patients is often suicide (52). A recent translational study examined TSPO in an infection-mediated animal model of schizophrenia. Higher levels of proinflammatory cytokines were found in brain regions that also showed lower TSPO expression as measured using immunohistochemistry (55), an observation that paralleled TSPO PET and cerebrospinal fluid data in patients (24). Importantly, microglia and astrocytes have been found to exist in both pro- and anti-inflammatory states (56,57), which cannot be differentiated by TSPO. Indeed, very recent in vitro data suggest that M1 (proinflammatory) macrophages and microglia may show lower TSPO expression in humans (58,59). The above-discussed literature, together with the results of our study, challenges the utility of TSPO as an exclusively proinflammatory marker in schizophrenia. Lower levels of TSPO could indicate a compensatory mechanism to a proinflammatory signal (55,60) or altered function of glial cells such as abnormal energy use (61). Because stimulation of TSPO has been shown to attenuate microglial activation in response to neuroinflammatory challenges (62–64), lower TSPO in psychosis could also indicate an inherent weaker anti-inflammatory response. These hypotheses all need to be addressed in future studies.
Because there is no brain region devoid of TSPO expression (65,66), metabolite-corrected arterial plasma measurements of radioligand concentration are necessary for accurate in vivo quantification of binding. To overcome variability that may be associated with the arterial measurements (27,67), relative measures of binding, such as distribution volume ratios (DVRs), have been proposed (22). Of the studies included in this meta-analysis, one study reported a significantly higher DVR in patients with schizophrenia and people at clinical risk for psychosis (22), whereas three studies showed no difference in schizophrenia (23–25). More recently, one study found no evidence of higher DVR in high-risk individuals compared with HCs (68). We chose not to include DVR in our analysis. The interpretation of patient–control differences obtained by dividing binding in a target region by that in a reference region is complicated by the possibility that there are alterations in specific binding in the reference region as well. In addition, the reliability of DVR for TSPO radioligands has been found to be low (69). Given the lack of a true reference region, VT is the most suitable outcome for TSPO quantification under the assumption that nondisplaceable binding does not differ between groups. Apart from glial cells, TSPO is also expressed in perivascular and endothelial cells (55,70) and under certain conditions also neurons (71). Further research is needed to evaluate the contribution of these components to the observation of lower levels of VT in schizophrenia. Finally, while there is as yet no published evidence showing an effect of the fraction of free radiotracer in plasma on brain VT for TSPO radioligands (72), it cannot be ruled out that potential patient–control differences in free radiotracer might contribute to the observed differences in VT. Of all the original studies included in this meta-analysis that measured free radiotracer (22–24), none found a significant difference between groups, suggesting that this factor did not have a major influence on the results.
In this IPD meta-analysis, the hierarchal statistical models allowed us to investigate the difference in TSPO levels between patients with psychosis and HCs across five different studies. The IPD approach offers many advantages over traditional, aggregated meta-analysis (73). In this study specifically, for example, it allowed us to examine the effect of medication, investigate correlations between VT and clinical measures, and control for potential cofounders such as gender, all of which would not have been possible if effect sizes had only been extracted from literature. By including only studies employing second-generation radiotracers and reporting the standard outcome measure VT, the analysis fulfills the precondition of meta-analytical models that outcomes should stem from the same underlying distribution of effects. Synthesizing data in this way, we were able to overcome the critical limitation of small sample sizes in the individual reports. Despite this, the total number of included subjects did not allow for investigations of specific subgroups such as different disease stages.
Conclusions
The current study shows that TSPO levels are lower across several brain regions in patients with first-episode psychosis and schizophrenia compared with HCs, suggesting an altered function, or reduced density, of immune and glial cells. Further work is needed to assess the exact biological meaning of these changes using both clinical and translational studies.
Supplementary Material
ACKNOWLEDGMENTS
This work is supported by funds from the Swedish Research Council (Grant No. 523–2014-3467 to SC) and Stockholm County Council (to SC), funding from PRIMA Barn- och Vuxenpsykiatri (to KC); funding from Research Councils UK (Grant No. MC-A656–5QD30 to ODH), Maudsley Charity (Grant No. 666 to ODH), Brain and Behavior Research Foundation (to ODH), Wellcome Trust (Grant No. 094849/Z/10/Z to ODH), and the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London (to ODH); funding from the Medical Research Council and King’s College London (to AHA); funding from U.S. Department of Defense (Grant No. GW130098 to MGP); and funding from the National Institutes of Health (Grant No. R01 MH100043 to RM). The funders had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; in the preparation, review, and approval of the manuscript; or in the decision to submit the manuscript for publication.
PP-S and SC conceived of the study, designed the study, wrote the study protocol, supervised the study, and carried out the literature search. PP-S, KC, MGP, JMC, YW, RM, PR, ODH, MV, AHA, and SC aided in the acquisition and quality control of data. PP-S and GJM performed the statistical analyses. PP-S and SC drafted the manuscript. All authors revised the manuscript for intellectual content and approved the final version.
We thank Yong Du and Sina Hafizi for helpful comments on the manuscript. The preregistration study and analysis protocol together with all analysis codes can be found at https://github.com/pontusps/TSPO_psychosis.
SC has received grant support from AstraZeneca as a coinvestigator and has served as a one-off speaker for Otsuka and Lundbeck. SC’s spouse is an employee of Swedish Orphan Biovitrum. ODH has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organized by AstraZeneca, Autifony, Bristol-Myers Squibb, Eli Lilly, Heptares, Janssen, Lundbeck, Leyden Delta, Otsuka, Servier, Sunovion, Rand, and Roche. RM has received a one-time speaking fee from Otsuka and Lundbeck.
Footnotes
DISCLOSURES
All other authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2018.02.1171.
Contributor Information
Pontus Plavén-Sigray, Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institutet and Stockholm Health Care Services, Stockholm County Council, Stockholm, Sweden.
Granville J. Matheson, Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institutet and Stockholm Health Care Services, Stockholm County Council, Stockholm, Sweden
Karin Collste, Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institutet and Stockholm Health Care Services, Stockholm County Council, Stockholm, Sweden.
Abhishekh H. Ashok, Institute of Psychiatry, Psychology, & Neuroscience, King’s College London; Medical Research Council London Institute of Medical Sciences, Faculty of Medicine, Imperial College London, London, United Kingdom; Hammersmith Hospital, and Institute of Clinical Sciences, Faculty of Medicine, Imperial College London, London, United Kingdom
Jennifer M. Coughlin, Department of Psychiatry and Behavioral Sciences, Johns Hopkins Medical Institutions, Baltimore, Maryland; Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins Medical Institutions, Baltimore, Maryland
Oliver D. Howes, Institute of Psychiatry, Psychology, & Neuroscience, King’s College London; Medical Research Council London Institute of Medical Sciences, Faculty of Medicine, Imperial College London, London, United Kingdom; Hammersmith Hospital, and Institute of Clinical Sciences, Faculty of Medicine, Imperial College London, London, United Kingdom
Romina Mizrahi, Department of Psychiatry, University of Toronto, Toronto, Ontario, Canada..
Martin G. Pomper, Department of Psychiatry and Behavioral Sciences, Johns Hopkins Medical Institutions, Baltimore, Maryland; Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins Medical Institutions, Baltimore, Maryland
Pablo Rusjan, Department of Psychiatry, University of Toronto, Toronto, Ontario, Canada..
Mattia Veronese, Department of Neuroimaging, King’s College London.
Yuchuan Wang, Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins Medical Institutions, Baltimore, Maryland.
Simon Cervenka, Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institutet and Stockholm Health Care Services, Stockholm County Council, Stockholm, Sweden.
REFERENCES
- 1.Arias I, Sorlozano A, Villegas E, de Dios Luna J, McKenney K, Cervilla J, et al. (2012): Infectious agents associated with schizophrenia: A meta-analysis. Schizophr Res 136:128–136. [DOI] [PubMed] [Google Scholar]
- 2.Ripke S, Neale BM, Pers TH, Julià A, Kahn RS, Kalaydjieva L, , Schizophrenia Working Group of the Psychiatric Genomics Consortium. (2014): Biological insights from 108 schizophrenia-associated genetic loci. Nature 511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Upthegrove R, Manzanares-Teson N, Barnes NM (2014): Cytokine function in medication-naive first episode psychosis: A systematic review and meta-analysis. Schizophr Res 155:101–108. [DOI] [PubMed] [Google Scholar]
- 4.Akhondzadeh S, Tabatabaee M, Amini H, Abhari SAA, Abbasi SH, Behnam B (2007): Celecoxib as adjunctive therapy in schizophrenia: A double-blind, randomized and placebo-controlled trial. Schizophr Res 90:179–185. [DOI] [PubMed] [Google Scholar]
- 5.Müller N, Krause D, Dehning S, Musil R, Schennach-Wolff R, Obermeier M, et al. (2010): Celecoxib treatment in an early stage of schizophrenia: Results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res 121:118–124. [DOI] [PubMed] [Google Scholar]
- 6.Rapaport MH, Delrahim KK, Bresee CJ, Maddux RE, Ahmadpour O, Dolnak D (2005): Celecoxib augmentation of continuously ill patients with schizophrenia. Biol Psychiatry 57:1594–1596. [DOI] [PubMed] [Google Scholar]
- 7.Ory D, Planas A, Dresselaers T, Gsell W, Postnov A, Celen S, et al. (2015): PET imaging of TSPO in a rat model of local neuroinflammation induced by intracerebral injection of lipopolysaccharide. Nucl Med Biol 42:753–761. [DOI] [PubMed] [Google Scholar]
- 8.Toth M, Little P, Arnberg F, Mulder J, Halldin C, Ha J, Holmin S (2016): Acute neuroinflammation in a clinically relevant focal cortical ischemic stroke model in rat: Longitudinal positron emission tomography and immunofluorescent tracking. Brain Struct Funct 221:1279–1290. [DOI] [PubMed] [Google Scholar]
- 9.Venneti S, Lopresti BJ, Wiley CA (2013): Molecular imaging of microglia/macrophages in the brain. Glia 61:10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doorduin J, De Vries EFJ, Willemsen ATM, De Groot JC, Dierckx RA, Klein HC (2009): Neuroinflammation in schizophrenia-related psychosis: A PET study. J Nucl Med 50:1801–1807. [DOI] [PubMed] [Google Scholar]
- 11.Van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, et al. (2008): Microglia activation in recent-onset schizophrenia: A quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry 64:820–822. [DOI] [PubMed] [Google Scholar]
- 12.Jučaite A, Cselényi Z, Arvidsson A, Åhlberg G, Julin P, Varnäs K, et al. (2012): Kinetic analysis and test–retest variability of the radioligand [11C](R)-PK11195 binding to TSPO in the human brain—A PET study in control subjects. EJNMMI Res 2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slifstein M, Laruelle M (2001): Models and methods for derivation of in vivo neuroreceptor parameters with PET and SPECT reversible radiotracers. Nucl Med Biol 28:595–608. [DOI] [PubMed] [Google Scholar]
- 14.Varnäs K, Varrone A, Farde L (2013): Modeling of PET data in CNS drug discovery and development. J Pharmacokinet Pharmacodyn 40:267–279. [DOI] [PubMed] [Google Scholar]
- 15.Holmes SE, Hinz R, Drake RJ, Gregory CJ, Conen S, Matthews JC, et al. (2016): In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: A [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry 21:1672–1679. [DOI] [PubMed] [Google Scholar]
- 16.van der Doef TF, de Witte LD, Sutterland AL, Jobse E, Yaqub M, Boellaard R, et al. (2016): In vivo (R)-[11C]PK11195 PET imaging of 18kDa translocator protein in recent onset psychosis. NPJ Schizophr 2:16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Biase MA, Zalesky A, O’Keefe G, Laskaris L, Baune BT, Weickert CS, et al. (2017): PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl Psychiatry 7:e1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita M, Kobayashi M, Ikawa M, Gunn RN, Rabiner EA, Owen DR, et al. (2017): Comparison of four 11C-labeled PET ligands to quantify translocator protein 18 kDa (TSPO) in human brain: (R)-PK11195, PBR28, DPA-713, and ER176—Based on recent publications that measured specific-to-non-displaceable ratios. EJNMMI Res 7:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi M, Jiang T, Telu S, Zoghbi SS, Gunn RN, Rabiner EA, et al. (2018): 11C-DPA-713 has much greater specific binding to translocator protein 18 kDa (TSPO) in human brain than 11C-(R)-PK11195. J Cereb Blood Flow Metab 38:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreisl WC, Fujita M, Fujimura Y, Kimura N, Jenko KJ, Kannan P, et al. (2010): Comparison of [11C]-(R)-PK 11195 and [11C]PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: Implications for positron emission tomographic imaging of this inflammation biomarker. NeuroImage 49:2924–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson AA, Garcia A, Parkes J, McCormick P, Stephenson KA, Houle S, Vasdev N (2008): Radiosynthesis and initial evaluation of [18F]-FEPPA for PET imaging of peripheral benzodiazepine receptors. Nucl Med Biol 35:305–314. [DOI] [PubMed] [Google Scholar]
- 22.Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, et al. (2016): Microglial activity in people at ultra high risk of psychosis and in schizophrenia: An [11C]PBR28 PET brain imaging study. Am J Psychiatry 173:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collste K, Plavén-Sigray P, Fatouros-Bergman H, Victorsson P, Schain M, Forsberg A, et al. (2017): Lower levels of the glial cell marker TSPO in drug-naive first-episode psychosis patients as measured using PET and [11C]PBR28. Mol Psychiatry 22:850–856. [DOI] [PubMed] [Google Scholar]
- 24.Coughlin JM, Wang Y, Ambinder EB, Ward RE, Minn I, Vranesic M, et al. (2016): In vivo markers of inflammatory response in recent-onset schizophrenia: A combined study using [11C]DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry 6:e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hafizi S, Tseng HH, Rao N, Selvanathan T, Kenk M, Bazinet RP, et al. (2017): Imaging microglial activation in untreated first-episode psychosis: A PET study with [18F]FEPPA. Am J Psychiatry 174:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenk M, Selvanathan T, Rao N, Suridjan I, Rusjan P, Remington G, et al. (2015): Imaging neuroinflammation in gray and white matter in schizophrenia: An in-vivo PET study with [18F]-FEPPA. Schizophr Bull 41:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collste K, Forsberg A, Varrone A, Amini N, Aeinehband S, Yakushev I, et al. (2016): Test–retest reproducibility of [11C]PBR28 binding to TSPO in healthy control subjects. Eur J Nucl Med Mol Imaging 43:173–183. [DOI] [PubMed] [Google Scholar]
- 28.Kreisl WC, Jenko KJ, Hines CS, Lyoo CH, Corona W, Morse CL, et al. (2013): A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab 33:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, et al. (2012): An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab 32:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owen DRJ, Gunn RN, Rabiner EA, Bennacef I, Fujita M, Kreisl WC, et al. (2011): Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J Nucl Med 52:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drzyzga Ł, Obuchowicz E, Marcinowska A, Herman ZS (2006): Cytokines in schizophrenia and the effects of antipsychotic drugs. Brain Behav Immun 20:532–545. [DOI] [PubMed] [Google Scholar]
- 32.Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, Tierney JF (2015): Preferred reporting items for a systematic review and meta-analysis of individual participant data: The PRISMA-IPD statement. JAMA 313:1657–1665. [DOI] [PubMed] [Google Scholar]
- 33.Maeda J, Suhara T, Zhang M, Okauchi T, Yasuno F, Ikoma Y, et al. (2004): Novel peripheral benzodiazepine receptor ligand [11C]DAA1106 for PET: An imaging tool for glial cells in the brain. Synapse 52:283–291. [DOI] [PubMed] [Google Scholar]
- 34.Vehtari A, Gelman A, Gabry J (2017): Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat Comput 27:1413–1432. [Google Scholar]
- 35.Jeffreys H (1961): Theory of Probability, 3rd ed. Oxford, UK: Oxford University Press. [Google Scholar]
- 36.Kass RE, Raftery AE (1995): Bayes factors. J Am Stat Assoc 90:773–795. [Google Scholar]
- 37.Morey RD, Wagenmakers E-J (2014): Simple relation between Bayesian order-restricted and point-null hypothesis tests. Statist Probab Lett 92:121–124. [Google Scholar]
- 38.Cohen J (1998): Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ: Lawrence Erbaum. [Google Scholar]
- 39.Dienes Z (2014): Using Bayes to get the most out of non-significant results. Front Psychol 5:781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gelman A (2006): Prior distributions for variance parameters in hierarchical models (comment on article by Browne and Draper). Bayesian Anal 1:515–534. [Google Scholar]
- 41.Carpenter B, Gelman A, Hoffman M, Lee D, Goodrich B, Betancourt M, et al. (2017): Stan: A probabilistic programming language. J Stat Softw 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buerkner P-C (2016): brms: An R package for Bayesian multilevel models using Stan. J Stat Softw 80:1–28. [Google Scholar]
- 43.Bates D, Mächler M, Bolker B, Walker S (2015): Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. [Google Scholar]
- 44.Kalk NJ, Owen DR, Tyacke RJ, Reynolds R, Rabiner EA, Lingford-Hughes AR, Parker CA (2013): Are prescribed benzodiazepines likely to affect the availability of the 18 kDa translocator protein (TSPO) in PET studies? Synapse 67:909–912. [DOI] [PubMed] [Google Scholar]
- 45.Monji A, Kato TA, Mizoguchi Y, Horikawa H, Seki Y, Kasai M, et al. (2013): Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry 42:115–121. [DOI] [PubMed] [Google Scholar]
- 46.Danovich L, Veenman L, Leschiner S, Lahav M, Shuster V, Weizman A, Gavish M (2008): The influence of clozapine treatment and other antipsychotics on the 18 kDa translocator protein, formerly named the peripheral-type benzodiazepine receptor, and steroid production. Eur Neuropsychopharmacol 18:24–33. [DOI] [PubMed] [Google Scholar]
- 47.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B (2011): Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol Psychiatry 70:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howes OD, McCutcheon R (2017): Inflammation and the neural diathesis-stress hypothesis of schizophrenia: A reconceptualization. Transl Psychiatry 7:e1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mizrahi R (2016): Social stress and psychosis risk: Common neurochemical substrates? Neuropsychopharmacology 41:666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laskaris LE, Di Biase MA, Everall I, Chana G, Christopoulos A, Skafidas E, et al. (2016): Microglial activation and progressive brain changes in schizophrenia. Br J Pharmacol 173:666–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trépanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP (2016): Postmortem evidence of cerebral inflammation in schizophrenia: A systematic review. Mol Psychiatry 21:1009–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Kesteren C, Gremmels H, de Witte LD, Hol EM, Van Gool AR, Falkai PG, et al. (2017): Immune involvement in the pathogenesis of schizophrenia: A meta-analysis on postmortem brain studies. Transl Psychiatry 7:e1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lavisse S, Guillermier M, Hérard A-S, Petit F, Delahaye M, Van Camp N, et al. (2012): Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. J Neurosci 32:10809–10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurumaji A, Wakai T, Toru M (1997): Decreases in peripheral-type benzodiazepine receptors in postmortem brains of chronic schizophrenics. J Neural Transm 104:1361–1370. [DOI] [PubMed] [Google Scholar]
- 55.Notter T, Coughlin JM, Gschwind T, Weber-Stadlbauer U, Wang Y, Kassiou M, et al. (2018): Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol Psychiatry 23:323–334. [DOI] [PubMed] [Google Scholar]
- 56.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. (2017): Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prinz M, Priller J (2014): Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Nat Rev Neurosci 15:300–312. [DOI] [PubMed] [Google Scholar]
- 58.Narayan N, Mandhair H, Smyth E, Dakin SG, Kiriakidis S, Wells L, et al. (2017): The macrophage marker translocator protein (TSPO) is down-regulated on pro-inflammatory “M1” human macrophages. PLoS One 12:e0185767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Owen DR, Narayan N, Wells L, Healy L, Smyth E, Rabiner EA, et al. (2017): Pro-inflammatory activation of primary microglia and macrophages increases 18 kDa translocator protein expression in rodents but not humans. J Cereb Blood Flow Metab 37:2679–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forsberg A, Cervenka S, Jonsson Fagerlund M, Rasmussen LS, Zetterberg H, Erlandsson Harris H, et al. (2017): The immune response of the human brain to abdominal surgery. Ann Neurol 81:572–582. [DOI] [PubMed] [Google Scholar]
- 61.Banati RB, Middleton RJ, Chan R, Hatty CR, Kam WW-Y, Quin C, et al. (2014): Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nat Commun 5:5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryu JK, Choi HB, McLarnon JG (2005): Peripheral benzodiazepine receptor ligand PK11195 reduces microglial activation and neuronal death in quinolinic acid-injected rat striatum. Neurobiol Dis 20:550–561. [DOI] [PubMed] [Google Scholar]
- 63.Leaver KR, Reynolds A, Bodard S, Guilloteau D, Chalon S, Kassiou M (2012): Effects of translocator protein (18 kDa) ligands on microglial activation and neuronal death in the quinolinic-acid-injected rat striatum. ACS Chem Neurosci 3:114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang W, Zhang L, Zhang X, Xue R, Li L, Zhao W, et al. (2016): Lentiviral-mediated overexpression of the 18 kDa translocator protein (TSPO) in the hippocampal dentate gyrus ameliorates LPS-induced cognitive impairment in mice. Front Pharmacol 7:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doble A, Malgouris C, Daniel M, Daniel N, Imbault F, Basbaum A, et al. (1987): Labelling of peripheral-type benzodiazepine binding sites in human brain with [3H]PK 11195: Anatomical and subcellular distribution. Brain Res Bull 18:49–61. [DOI] [PubMed] [Google Scholar]
- 66.Owen DR, Guo Q, Kalk NJ, Colasanti A, Kalogiannopoulou D, Dimber R, et al. (2014): Determination of [11C]PBR28 binding potential in vivo: A first human TSPO blocking study. J Cereb Blood Flow Metab 34:989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park E, Gallezot J-D, Delgadillo A, Liu S, Planeta B, Lin S-F, et al. (2015): 11C-PBR28 imaging in multiple sclerosis patients and healthy controls: Test–retest reproducibility and focal visualization of active white matter areas. Eur J Nucl Med Mol Imaging 42:1081–1092. [DOI] [PubMed] [Google Scholar]
- 68.Hafizi S, Da Silva T, Gerritsen C, Kiang M, Bagby RM, Prce I, et al. (2017): Imaging microglial activation in individuals at clinical high risk for psychosis: An in vivo PET study with [18F]FEPPA. Neuro-psychopharmacology 42:2474–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matheson G, Plavén-Sigray P, Forsberg A, Varrone A, Farde L, Cervenka S (2017): Assessment of simplified ratio-based approaches for quantification of PET [11C]PBR28 data. EJNMMI Res 7:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Veronese M, Reis Marques T, Bloomfield PS, Rizzo G, Singh N, Jones D, et al. (2018): Kinetic modelling of [11C]PBR28 for 18kDa translocator protein PET data: A validation study of vascular modelling in the brain using XBD173 and tissue analysis. J Cereb Blood Flow Metab 38:1227–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang M, Liu J, Zhou M-M, Wu H, Hou Y, Li Y-F, et al. (2016): Elevated neurosteroids in the lateral thalamus relieve neuropathic pain in rats with spared nerve injury. Neurosci Bull 32:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cumming P, Burgher B, Patkar O, Breakspear M, Vasdev N, Thomas P, et al. (2017): Sifting through the surfeit of neuro-inflammation tracers. J Cereb Blood Flow Metab 38:204–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tudur Smith C, Marcucci M, Nolan SJ, Iorio A, Sudell M, Riley R, et al. (2016): Individual participant data meta-analyses compared with meta-analyses based on aggregate data. Cochrane Database Syst Rev 2016 Sep 6;9:MR000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Erp TGM, Preda A, Nguyen D, Faziola L, Turner J, Bustillo J, et al. (2014): Converting positive and negative symptom scores between PANSS and SAPS/SANS. Schizophr Res 152:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


