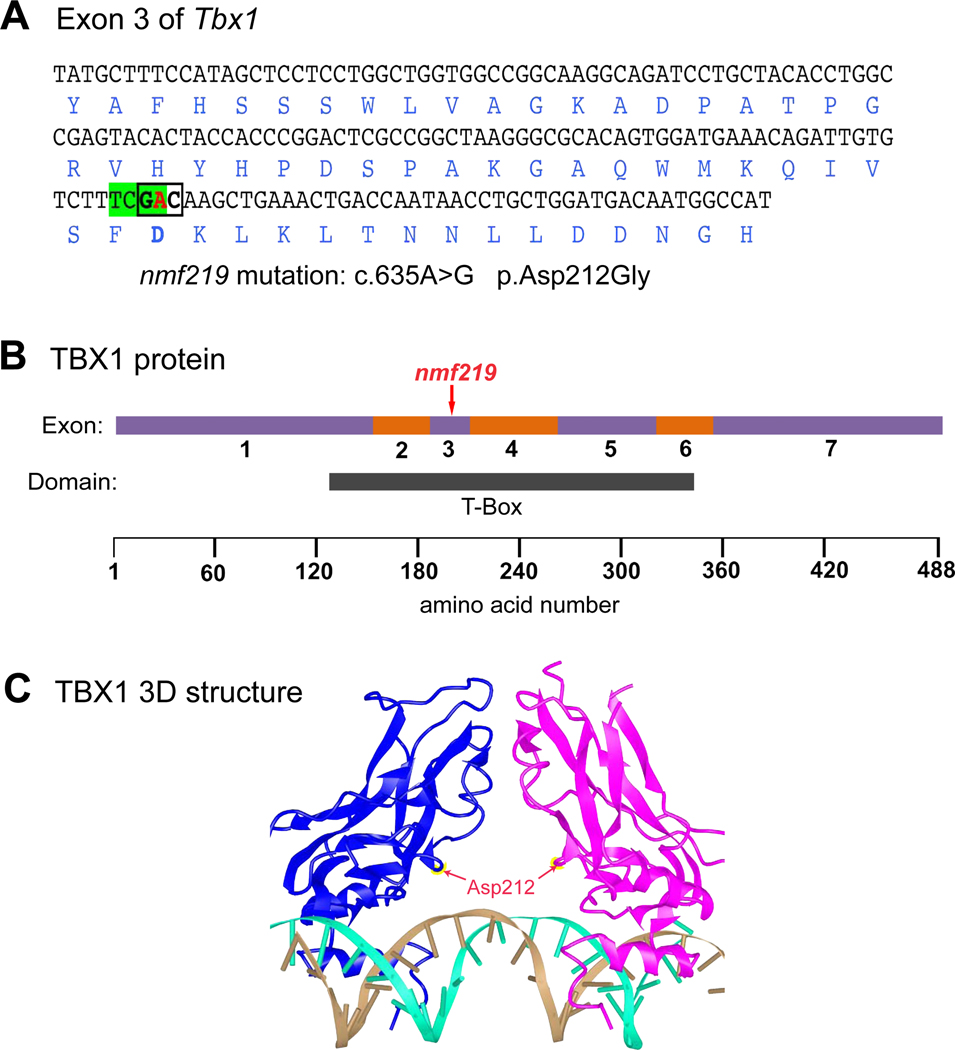
Figure 1. Molecular characterization and consequences of the Tbx1nmf219 mutation.
A. DNA sequence of Tbx1 exon 3 with encoded amino acids shown below in blue font. The adenine (A) nucleotide at coding position 653 of Tbx1 cDNA (shown in red) is changed to guanine (G) by the nmf219 mutation (c.635A>G), which changes the GAC codon (boldface, boxed) for aspartic acid (Asp) to glycine (Gly) at position 212 of the TBX1 protein (p.Asp212Gly). The mutation also destroys a TaqI restriction enzyme recognition site (TCGA, highlighted in green). B. Schematic diagram of the TBX1 protein with regions encoded by exons 1–7 depicted as alternating purple and orange bars. The nmf219 mutation occurs in exon 3, which encodes part of the T-box DNA binding domain, demarcated by the black bar below the protein diagram. Corresponding amino acid positions are shown at the bottom of the diagram. C. The 3D structure of the DNA-bound T-box domain of the human TBX1 protein. TBX1 binds DNA as a dimer; the individual monomers are shown in blue and magenta in the diagram. Red arrows point to the Asp212 residues of the monomers, which are mutated to Gly in nmf219 mutant mice. The two strands of the DNA helix are shown in gold and cyan. The 3D structure of the human protein was retrieved from the Molecular Modeling Database (MMDB) and provides an accurate proxy for the mouse protein because the 190 amino acid sequence of the T-box domain of the human TBX1 protein (aa 110–299, NP-542377) is identical to the 190 amino acid sequence of the T-box domain of the mouse TBX1 protein (aa 108–297, NP-035 662).