Abstract
The crustacean cardiac neuromuscular system is a useful model for studying how neural circuits generate behavior, as it is comprised of a simple ganglion containing nine neurons, yet acts as a robust central pattern generator. The crustacean heart is neurogenic, receiving input from neuropeptides. However, the specific effects of neuropeptides on cardiac output is not fully understood, and the large degree of comodulation between multiple neuropeptides makes studying these effects more challenging. To address this challenge, matrix-assisted laser desorption/ionization (MALDI) mass spectrometry (MS) imaging was used to localize neuropeptides within the cardiac ganglion (CG), providing information about the identity and localization of neuropeptides being present. CG extracts were also profiled using liquid chromatography (LC) coupled to tandem mass spectrometry (MS/MS) with a data independent acquisition (DIA) method, resulting in the confirmation of 316 neuropeptides. Two MS imaging (MSI) platforms were compared in order to provide comprehensive results, including a MALDI-Orbitrap instrument for high mass spectral resolution for accurate identifications and a MALDI-TOF/TOF for improved spatial resolution and sensitivity, providing more descriptive MS images. MS images for 235 putative neuropeptides were obtained, with the identification of 145 of these being confirmed by either complementary MS/MS data or accurate mass matching. The MSI studies demonstrate the sensitivity and power of MALDI-based in situ analytical strategy for unraveling the chemical complexity present in a small 9-cell neuronal system. The results of this study will enable more informative assays of the functions of neuropeptides within this important neural circuit.
Keywords: Neuropeptides, mass spectrometry imaging, crustacean, cardiac neurophysiology
Graphical Abstract

Introduction
Central pattern generators have long been studied in order to understand how neural circuits generate behavior. Crustaceans have been particularly useful for studying the chemical modulation of neural circuits because of their simplicity [1]. Circuits driving behavior in decapod crustaceans consist of a small number of easily-distinguishable neurons, making it possible to map how neurons connect to each other and form feedback loops [2]. Furthermore, the effect of substances modulating this activity, such as neuropeptides and neurotransmitters, can be more easily studied to determine how they affect the bursting patterns of specific neurons [3, 4].
The crustacean cardiac neuromuscular system is of great utility for studying circuit dynamics, as it is one of the simplest central pattern generators [5, 6]. Crustaceans have an open-circulatory system, in which tissues are bathed in hemolymph that is circulated by means of a single-chambered heart [7]. The heart’s cardiac neuromuscular system consists of the cardiac ganglion (CG) and surrounding muscular tissue. While the specific neuronal makeup of the CG varies, in Cancer borealis, it is comprised of 9 neurons, including 4 pacemaker neurons and 5 motor neurons. These neurons are connected in a manner to drive the heartbeat, signaling to the muscle tissue to drive contractions, pushing hemolymph throughout the rest of the crab [6].
As crustacean hearts are neurogenic, neuropeptides, along with neurotransmitters, are responsible for modulating the heartbeat. There have been numerous studies investigating the roles of specific neuropeptides on the CG of both crabs and lobsters, including those belonging to the following families: pyrokinin [8], allatostatin [9], tachykinin [6], and SIFamide [10]. These findings have unveiled how individual neuropeptides alter heart contractions, both amplitude and frequency, and how these effects change as a function of other factors such as concentration or temperature [8, 11, 12]. Studies have also illuminated the intricate complexity within neuropeptide families, as neuropeptides possessing common sequence motifs often elicit contrasting functions. For example, neuropeptides belonging to the pyrokinin family possess the common motif –FXPRLamide, but when 5 of these were tested for effect on heart activity, only one had an effect, increasing both contraction amplitude and frequency [8, 13]. Similarly, 3 isoforms of allatostatin C-type were found to elicit distinct cardiac outputs in lobster hearts despite their sequence similarity [9]. Immunohistology has also been employed in order to localize these neuropeptides to discrete areas of the ganglion [4, 9].
The results from these studies have been helpful in determining the functions of specific neuropeptide isoforms within the cardiac neuromuscular system. However, these studies have been limited to individual neuropeptides. It has been found that neuropeptides often co-modulate one another to elicit or alter functions within circuits [14]. This finding suggests that the function of neuropeptides studied in artificial saline may be different than in vivo because of the presence of other neuropeptides within or surrounding the ganglion. In order to fully understand the function of neuropeptides, their effects must be studied in relation to other co-modulating substances.
Currently, little is known about what neuropeptides are present in the C. borealis cardiac ganglion that may be affecting its activity. There have been extensive studies profiling and localizing the neuropeptides in other neural tissue, such as members of the stomatogastric nervous system, another robust central pattern generating circuit in the crab, as well as neurosecretory tissue that release neuropeptides into the heart, most notably the pericardial organs that are located laterally on either side of the heart [15-19]. However, there has not yet been an in-depth profiling of the neuropeptides present in the CG.
The purpose of this study is to implement a multifaceted MS method to image neuropeptides present in the C. borealis CG and localize these neuropeptides to general regions of the tissue. An MS imaging method was employed to localize these neuropeptides to discrete regions of this small tissue. A multi-platform approach was used to identify and localize neuropeptides, including matrix-assisted laser desorption ionization (MALDI) MS imaging on an Orbitrap MS for high mass spectral resolution and a time of flight (ToF) MS for high spatial resolution and speed for mapping these peptides, and LC-electrospray ionization (ESI)-MS/MS for confident identification. The combined approach enabled the identification of 316 neuropeptides across 19 families and the localization of 235 neuropeptides to areas of the CG that correlate with various cellular regions, demonstrating the powerful results of this method, both in terms of sensitivity and specificity, for studies of small, yet complex neuronal systems.
Materials and Methods
Chemicals and Materials
ACS-grade formic acid (FA) was purchased from Sigma-Aldrich (St. Louis, Mo, USA). Gelatin was purchased from Becton Dickinson (Franklin Lakes, NJ, USA). All other chemicals and solvents were purchased from Fisher Scientific (Pittsburgh, PA, USA). ACS-grade solvents were used for sample preparation, and Optima-grade solvents were used for MS analysis. Acidified methanol was prepared using 90/9/1 water/methanol/acetic acid. 10 μL C18 ziptips were purchased from Merck Millipore (Billerica, MA, USA).
Animal dissection, tissue collection, and sample preparation
Male Cancer borealis animals purchased from the Fresh Lobster Company, LLC (Gloucester, MA, USA) were housed in artificial seawater tanks maintained at 12-13°C with an alternating 12-hour light/dark cycle. Animals were allowed to adjust to their environment for a minimum of one week prior to use. For tissue dissection, animals were cold-anesthetized for 30 minutes on packed ice and dissected in chilled artificial crab saline (440 mM NaCl, 11 mM KCl, 26 mM MgCl2, 13 mM CaCl2, 11 mM Trizma base, 5 mM maleic acid, adjusted to pH 7.45 with NaOH).
For LC-MS/MS experiments, tissues were collected and placed in chilled acidified methanol (90/9/1 methanol/water/acetic acid). Tissues were manually homogenized with a glass homogenizer in chilled acidified methanol, sonicated in a bath sonicator, and centrifuged at 16.1 rcf for 10 minutes. The supernatant was collected, acidified methanol was added to the pellet, and the process was repeated for two more extractions. The combined supernatants were evaporated in a speedvac on medium heat and reconstituted in 10 μL of 0.1% FA in water. The samples were then desalted using C18 ziptips following package instructions, and the elutions were evaporated in the speedvac on medium heat. Extracts from 12 CGs were pooled for subsequent LC-MS/MS analysis.
For imaging of the CG and PO, tissues were collected, dipped in water to desalt, placed on a plain glass or indium tin oxide (ITO)-coated glass microscope slide (Bruker, Billerica, MA, USA), and dried in a dessicator box. For imaging of the brain, the tissue was collected, dipped in water to desalt, and embedded in gelatin and flash frozen on dry ice. The brain was then sectioned at 12 μm thickness and placed on a plain glass or ITO-coated glass microscope slides. Each tissue was coated with 12 passes of 40 mg/mL 2,5-dihydroxy benzoic acid (DHB) using an automated TM sprayer (HTX Technologies LLC, Chapel Hill, NC, USA).
LC-MS/MS analysis
The CG extract was reconstituted in 15 μL of 0.1% FA in water and was analyzed on a Thermo Scientific Q Exactive HF instrument coupled to an online Dionex UltiMate 3000 nanoLC (Thermo Scientific, Bremen, Germany). A 15-cm self-packed C18 column (75 μm internal diameter, 1.7 μm particle size) was used for LC separation. Mobile phases consisted of water with 0.1% FA (A) and acetonitrile with 0.1% FA (B). The flow rate was set to 0.300 μL/min, and a μL injection volume was used. The LC run consisted of a 120-minute gradient as follows: 0-1 min 3-10% B; 1-90 min 10-35% B; 90-92 min 35-95% B; 92-102 min 95% B; 102-105 min 95-3% B; 105-120 min 3% B. For MS analysis, a data independent acquisition (DIA) method was utilized, as has previously been optimized [19]. Positive ESI was used with a collection energy of 30 eV, 70,000 resolution for MS scans, and 17,500 resolution for MS/MS scans. The MS scan range was 400 to 800 m/z with an isolation width of 20 m/z.
MALDI-MS analysis
MALDI-MS imaging was performed on a Thermo Scientific MALDI-LTQ-Orbitrap XL mass spectrometer (Thermo Scientific, Bremen, Germany) and a Bruker RapifleX MALDI Tissuetyper ToF/ToF (Bruker, Billerica, MA, USA). Optical images were acquired with an HP scanner and imported into each instrument’s tune page. The MALDI-Orbitrap mass spectrometer was equipped with a 337.1 nm, 60 Hz nitrogen laser and charge-coupled device camera (CCD) that were manufactured by Thermo Scientific (Thermo Scientific). Samples were analyzed in positive-ion mode with an m/z range of 500 to 2000, 20 kJ laser energy, and 75 μm raster size. Alignment of tissue and laser source was controlled using the LTQ software (Thermo Scientific), and instrument methods were created using Xcalibur software (Thermo Scientific). Each MS scan included 2 microscans, and a mass resolution of 60,000 was used. All MS scans were acquired in the Orbitrap mass analyzer. The RapifleX mass spectrometer was equipped with a smart-beam 3D 10 kHz laser. Samples were analyzed in positive-ion mode with an m/z range of 200 to 3200, 80% laser energy, and 20 μm raster size.
The MALDI-Orbitrap instrument was also used for spot analysis of an extract containing 12 pooled CG tissues. The sample was dissolved in 2.5 μL 0.1% FA, mixed with equal volume of 120 mg/mL DHB in 50/50 water/methanol (v/v) with 0.1% FA, and spotted on a MALDI target plate, 1 μL per spot, 5 spots total. Crystal positioning system (CPS) plate motion was used to acquire a total of 50 scans (2 microscans/scan) for each spot.
Data analysis
MS spectra from LC-MS/MS analysis were converted to the open-source mzXML file format using MSConvert [20] and processed with DIA-Umpire [21] using the default parameters. Output mgf files were converted to mzXML and processed with PEAKS 7.0 (Bioinformatics Solutions Inc., Waterloo, ON, Canada) [22, 23] using the following paramters for de novo sequencing: no enzyme cleavage specified, instrument orbi-orbi, HCD fragmentation, and precursor correction enabled. Modifications were set to include amidation, pyroglutamate, dehydration, and oxidation. All other parameters were set to the default. An in-house crustacean neuropeptide database was used for searches. MALDI-MS spot data was processed using accurate mass-matching to the same crustacean neuropeptide database using a mass error of +/− 5 ppm.
MALDI MS imaging files were converted into either imzML (Orbitrap) or img (RapifleX) format using vendor-specific software. Data were loaded into MSiReader, and images were generated of neuropeptides in the crustacean neuropeptide database [24]. The mass error was set to 5 ppm for Orbitrap data and 0.1 Da for RapifleX data. All images were normalized to the total ion current (TIC). For the RapifleX data of CG images, 5th order linear smoothing was applied.
Results and Discussion
The crustacean CG is an important circuit in the crustacean neuromuscular system that modulates the contractions of the heart, circulating hemolymph throughout the animal. As the heart is neurogenic, knowing what neuropeptides are present within the tissue and where they are localized provides important insight into how the rhythmic activity of the CG correlates with behavioral outputs. To address this gap in knowledge, this study used a multi-platform MS approach to profile the neuropeptide content within the tissue and localize neuropeptides to specific areas of the tissue, providing insight into their biological activity.
Comparison of MS imaging platforms
As the CG is a small tissue and localization of neuropeptides to regions of specific neuronal tissue was desired, spatial resolution was critical to gaining quality MS images. The Bruker RapifleX instrument offers high spatial resolution in addition to greater acquisition speeds. However, the mass resolution of the TOF mass analyzer is notably lower compared to Orbitrap instruments, making high-confidence mass measurements more difficult. Furthermore, neuropeptide analysis of crustacean tissue samples has never before been performed on a Rapiflex instrument. In order to ensure data quality, a comparison was made on the two MS imaging platforms used for neuropeptide analysis, MALDI-LTQ-Orbitrap XL and MALDI RapifleX MALDI Tissuetyper ToF/ToF.
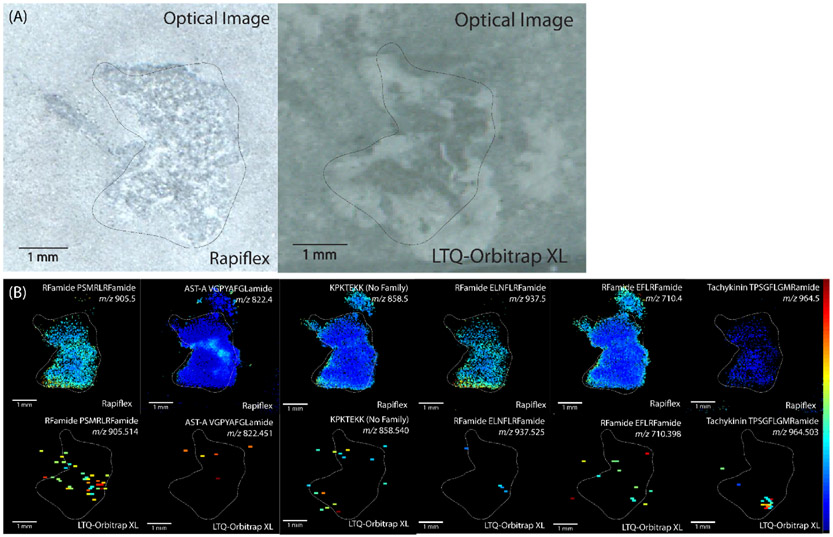
In order to compare the two platforms in their ability to detect neuropeptides in crustacean tissue, serial sections of crab brain tissue were analyzed on each platform. A total of 10 sections were analyzed, with 5 being on each respective platform. Brain tissue was used because the tissue is large enough to section such that technical replicates could be analyzed on each platform. Overall, 314 neuropeptides were putatively detected with the RapifleX TOF/TOF instrument, while only 19 were detected with the Orbitrap instrument. While the mass error tolerance was greater for the RapifleX than the Orbitrap, 14 neuropeptides were detected with both platforms, and these in general showed improvement in ion localization with the RapifleX because of the higher spatial resolution. Figure 1 shows examples of 6 neuropeptides detected with both platforms, demonstrating the improvement in ion images that was offered by the RapifleX. These results were observed for neuropeptides from a variety of families, i.e. sequence motifs, indicating that there was not bias toward specific neuropeptide families. While the Orbitrap MS imaging experiments show MS images as scattered signals, the improved spatial resolution of RapifleX images enable visualizing discrete localization within the tissue, indicating clustering of neuropeptides to specific ganglia within the brain.
Figure 1.
(A) Optical anatomical images coated with DHB matrix and (B) MALDI-MS images of C. borealis brain serial sections analyzed on a RapifleX TOF/TOF instrument (top) and LTQ-Orbitrap XL instrument (bottom). The comparison shows an improvement in spatial resolution for images acquired on the RapifleX. The image intensity was in general stronger for ions obtained on the RapifleX, but several showed stronger signal on the Orbitrap, such as tachykinin TPSGFLGMRamide (m/z 964.503).
Overall, however, the results appear to be in general agreement between the two platforms. The RFamide PSMRLRFamide and AST-A VGPYAFGLamide show broad distribution throughout the brain section, with greater signal in the center in the MS images acquired by Rapiflex, while the ion images acquired by Orbitrap were only able to detect the neuropeptide in the center, high-intensity area. Similarly, KPKTEKK, RFamide ELNFLRFamide, and RFamide EFLRFamide show greater signals on the outer edges of the tissue, with these being the only areas that the peptide is detectable with the Orbitrap. Tachykinin TPSGFLGMRamide shows a strong signal in a central area in the tissue that is in a similar location in both MS images, though the peptide images acquired by the Rapiflex platform show weaker intensity in the nearby area.
There are several factors that may contribute to the difference in number of neuropeptides detected between the two platforms, including MS acquisition speed, mass error tolerance of processed MS images, and sensitivity of mass analyzer. As the Rapiflex employs a ToF mass analyzer, the MS acquisition speed is much higher than that of the Orbitrap. Even with the smaller step size, the Rapiflex analyzed each brain section in less than one hour, while the Orbitrap required on average 2 hours per section. With a total of 5 sections analyzed, the overall experiment time for the Orbitrap instrument was approximately 10 hours, while the Rapiflex experiment time was less than 5 hours. The increased time that tissues were being kept under vacuum likely contributed to diminished detectability, particularly for the later sections. Additionally, images acquired with the two platforms were processed with different error tolerances. As TOF mass analyzers have lower resolving power and mass accuracy than Orbitraps, the error tolerance was set to 0.1 Da, which is considerably larger than that of the Orbitrap, set to 5 ppm. When Orbitrap images were processed with an error tolerance of 0.1 Da, the number of detected neuropeptides increased from 19 to 43, which indicates that mass tolerance contributes to some, but not all, of the discrepancies between the two platforms. This observation indicates the importance of a multifaceted approach for confirmation of identifications. Finally, the Rapiflex is in general more sensitive than the LTQ-Orbitrap instrument. As neuropeptides are present in low abundances in vivo, sensitivity is a critical factor in their detectability.
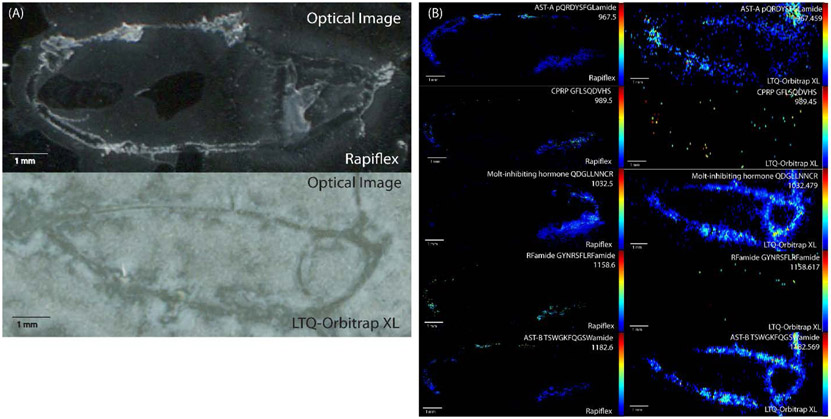
Pericardial organs (POs) were also used to compare the performance of each of the MS imaging platforms. While both the CG and PO are too thin to section for multiple replicates, the PO is a paired tissue, with one located on either side of the heart. Therefore, a comparison could be made in which POs from the same animal were analyzed on each platform, e.g. the left on the RapifleX and the right on the Orbitrap. As POs are neuroendocrine tissue that release neuropeptides into the heart of the crab, they contain a different cohort of neuropeptides than that is found in brain tissue, making it useful to use POs in addition to brain sections for testing and evaluating the platforms to ensure detectability of neuropeptides from different families. When analyzing neuropeptides from POs from the same crab, the RapifleX enabled the detection of 190 neuropeptides, while the Orbitrap detected 128 neuropeptides, with 52 of these neuropeptides being detected with both platforms. While the numbers were much more comparable to one another in the POs than in the brain tissue, the resulting RapifleX MS images in general had greater ion intensities than the Orbitrap MS images, indicating greater sensitivity of the RapifleX instrument, though several neuropeptides showed higher ion intensity in the Orbitrap MS images. Figure 2 shows a comparison of 5 neuropeptides identified with both platforms, showing the general improvement in spatial localization and ion images of most neuropeptides acquired from the RapifleX.
Figure 2.
(A) Optical anatomical images and (B) MALDI-MS images of C. borealis pericardial organs (POs) analyzed on a RapifleX TOF/TOF instrument (left) and LTQ-Orbitrap XL instrument (right). POs were collected from the same animal and the left was analyzed on the RapifleX and the right on the Orbitrap. The comparison shows an improvement in spatial resolution for images acquired on the RapifleX. The image intensity was stronger for some ions on the RapifleX and others on the Orbitrap.
Finally, CGs were imaged on both platforms in order to compare performance. CGs were collected from distinct crabs on the same day, with one being analyzed on the Orbitrap and one on the RapifleX. Again, the RapifleX instrument demonstrated superior performance, with 119 neuropeptides putatively detected, while the Orbitrap enabled the detection of only 24 neuropeptides. Based on the comparative results, it was determined that the sensitivity and spatial resolution offered by the RapifleX provided better performance for imaging the CG, while the MALDI Orbitrap XL platform would be used for complementary analysis to validate putative identifications due to its higher mass spectral resolution and accurate mass measurement.
Localization of neuropeptides within the cardiac ganglion
The cardiac ganglion consists of 9 neurons distributed asymmetrically across a tissue approximately 5 mm in length. The posterior side of the tissue connects to the posterior artery and extends to a T-shaped branch. Near this branch are 4 small pacemaker neurons that are electrically coupled and provide excitatory synaptic inputs to the 5 motor neurons. The motor neurons are located throughout the rest of the tissue, with 2 on the posterior side, near the pacemaker neurons. The anterior side of the CG branches into a Y shape, with 2 motor neurons on one side and 1 on the other side. The motor neurons work to produce depolarizing excitatory postsynaptic potentials on the heart muscle cells, thus driving the rhythmic contractions of the heart. The bursting of these neurons is controlled by Ca2+, K+ , and neuropeptide modulators [4]. By determining which neuropeptides are localized to specific regions of the ganglion, insight into how these neuropeptides modulate heart activity can be obtained.
A total of 235 neuropeptides were detected in 3 biological replicates of MS imaging of the CG, comprising 24 neuropeptide families. Confirmation of these identifications was performed by analyzing CG extract with MALDI-MS and LC-ESI-MS/MS. Of the 235 identifications, 104 were confirmed with MS/MS and 65 with accurate mass matching within a 5 ppm mass tolerance on the Orbitrap platforms. Table S1 lists all the neuropeptides identified in the CG with MSI, along with the method of confirmation for identification.
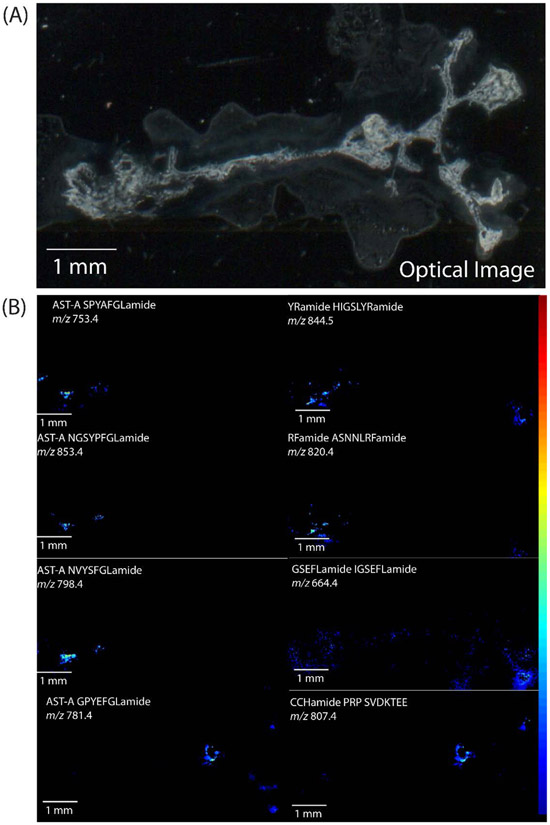
MS images of the distribution of several neuropeptides are shown in Figure 3, along with the optical image of the tissue. The neuropeptides shown exhibit localization patterns that correlate with the general location of neuron types within the tissue. For example, the neuropeptide A-type allatostatin SPYAFGLamide (m/z 753.4) and RFamide ASNNLRFamide (m/z 820.4) appear to be localized to the area containing posterior cells, including both the pacemaker and motor neurons in this region, while two A-type allatostatins, NVYSFGLamide (m/z 798.4) and NGSYPFGLamide (m/z 853.4), appear to be more concentrated to the area of the pacemaker neurons, with a small amount in the area of the posterior motor neurons. As RFamides have been shown to have excitatory functions and allatostatin peptides elicit inhibitory effects, their localization here indicates that these neuropeptides may also serve modulatory roles in the CG. The peptide HIGSLYRamide (m/z 844.5) is also localized to the posterior region of the CG, but is more broadly distributed, indicating potential presence in the axons of the neurons as well. While the functional role of HIGSLYRamide is not currently known, it has been widely detected throughout other tissues with previous MS studies, indicating it may have a variety of functions, both locally and as a circulating hormone [40-42]. There are also neuropeptides detected that are localized predominantly on the anterior side of the CG in the general area where the anterior motor neurons are located, including A-type allatostatin GPYEFGLamide (m/z 781.4), CCHamide precursor-related peptide SVDKTEE (m/z 807.4), and IGSEFLamide (m/z 664.4). While these three peptides have been detected in the area of the anterior motor neurons, they have distinct localization patterns from each other, indicating that they may all serve modulatory functions in these neurons, but their specific functions may differ from one another. Overall, the results here suggest that these neuropeptides may have a role in modulating CG outputs, either from within the neurons of the CG or transported from elsewhere, based on the observation of discrete localization patterns. However, additional immunocytochemistry experiments are necessary to determine the specific location of these neuropeptides, either within motor or pacemaker neurons or in terminals of various modulatory neurons and endocrine cells that enter the ganglion from other sources, such as the POs.
Figure 3.
(A) Optical anatomical image and (B) MALDI-MS images of C. borealis cardiac ganglion acquired on the RapifleX TOF/TOF instrument. Images represent neuropeptides localized to distinct regions of the tissue.
Profiling results
The MS imaging results shown contain only MS1 data reporting detection based on the intact mass of neuropeptides. In order to ensure confident identification of these neuropeptides, MS/MS spectra are required that can be used to gain sequence information about the neuropeptides. While in situ MS/MS collected is highly useful for identifying neuropeptides on-tissue, the small size and low-abundance of neuropeptides in the CG makes this a challenging task, as sample is very limited. Instead, in order to validate the neuropeptides localized in the CG, LC-MS/MS was used on tissues collected and pooled from 12 animals. The tissues were extracted, pooled together, and analyzed with LC-ESI-MS/MS using nanoLC coupled to an Orbitrap mass spectrometer with a nanoESI source. As the tissue contains only 9 neurons, the in vivo concentration of neuropeptides was expected to be low. In order to detect more low-abundance neuropeptides within the tissue, a DIA method was employed, as DIA has been previously demonstrated to improve the detection of neuropeptides in crustacean tissue [19]. The CG contains lipids and other interfering species that are present in high abundances. In contrast, if a conventional data dependent acquisition (DDA) method is implemented, in which only the top most abundant ions are selected for fragmentation, then fewer neuropeptides would be selected for fragmentation, resulting in a smaller number of identifications.
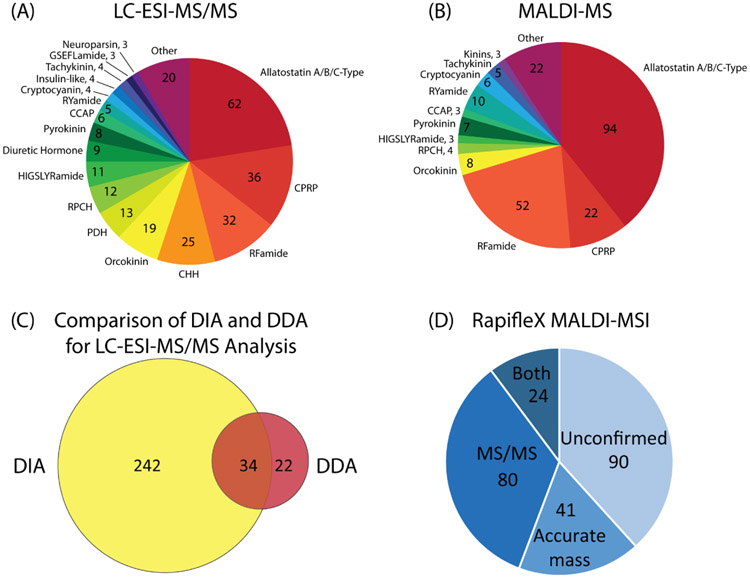
To demonstrate this difference, the same CG sample was analyzed with a top-10 DDA method, and the number of neuropeptides was compared. With the DDA method, 56 neuropeptides were identified, a considerably high number for such a small neuronal ganglion containing only nine neurons. However, with the DIA method, a total of 316 neuropeptides were identified, approximately six-fold improvement from the DDA method, including most of the neuropeptides identified with DDA. The drastic improvement in identification number is attributed to the ability to obtain fragment ions for all species in the sample spanning the given mass range of 400 to 800 m/z, regardless of relative abundance, as long as the abundance is above the limit of detection of the instrument. Figure 4C shows the overlap of neuropeptides identified with DIA and DDA methods, with results being similar to those found previously in brain and pericardial organ tissue from C. borealis [19].
Figure 4.
Representation of neuropeptides identified in the cardiac ganglion (CG), including the distribution of families neuropeptides belonged to that were identified with (A) LC-ESI-MS/MS with DIA analysis and (B) MALDI-MS with accurate mass matching. Also shown is (C) a comparison of two LC-MS/MS acquisition methods, DIA and DDA, showing the superior performance of DIA for the current analysis, and (D) a breakdown of the validation of neuropeptides detected with MSI on the RapifleX instrument, including those with confident MS/MS spectra, high-resolution accurate masses matching to the known database, and those with neither, listed as unconfirmed identifications.
In addition to LC-ESI-MS/MS analysis, analysis of extracts with MALDI-MS was also employed. Numerous previous studies have shown the complementary nature of ESI and MALDI as ionization methods [25-27]. MALDI was used here to identify additional neuropeptides that may not be easily detectable with ESI. By directly analyzing tissue extract from 12 pooled CGs, 239 neuropeptides were identified, a similar number to that identified by ESI. The lower number is likely due to signal suppression within the sample, as no separation step was included prior to MALDI MS analysis.
With the LC-ESI-MS/MS DIA method, a total of 316 neuropeptides were identified, belonging to 31 different neuropeptide families. With the MALDI-MS method, 239 neuropeptides were identified over 23 neuropeptide families. Of these neuropeptides, 49 were detected in both platforms, and the representation of neuropeptides within each family were similar between each, as shown in the pie charts in Figure 4A and 4B. These numbers are considerably large for a small, relatively simple tissue, indicating the underlying molecular complexity within the circuitry involved in this system. The breakdown of identification confirmations for all putative assignments made with MS imaging is shown in the pie chart in Figure 4D.
Neuropeptides possess common motifs that are characteristic of different groups, or families. The most commonly represented neuropeptide families in the CG are allatostatin, RFamide, crustacean hyperglycemic hormone (CHH) and its precursor related peptide (CPRP), orcokinin, and pyrokinin. Allatostatins are divided into three types: A-type, B-type, and C-type, all of which have been found to have inhibitory effects on neural circuits [28, 29]. The physiological effects of C-type allatostatins have already been characterized in the cardiac neuromuscular system, most causing a decrease in heart rate [9]. Allatostatin peptides have also been commonly identified in previous studies of the pericardial organs, which are located laterally on either side of the heart and are believed to release neuropeptides into the pericardial sinus [17, 30]. Therefore, it is expected that this family would be highly represented within the CG as well. Neuropeptides from the CHH superfamily have been implicated in numerous functions, including metabolism, ion transport, uptake of water, reproduction, and molting, mostly serving as endocrine signaling agents [31, 32]. As heart rate has been found to change with other physiological functions such as feeding, it is possible that CHH could also act as a signaling agent for cardiac output as well. While little is known about the function of CPRPs, they originate from the same precursors as CHH, so it is not surprising that they are present in the CG with CHH peptides. RFamide neuropeptides are broadly distributed throughout the nervous and neuroendocrine systems, functioning locally as autocrines and paracrines, as well as long range as circulating hormones [33-35]. Numerous RFamide neuropeptides have been found to modulate cardiac activity, and given the large number of RFamide peptide isoforms present in the CG, it is likely that additional RFamide neuropeptides play a physiological role in this tissue [36, 37]. The large presence of orcokinin neuropeptides in the CG is unexpected, as these neuropeptides are most frequently detected in nervous system tissue, such as the brain, and they have been implicated in modulating hindgut contractions and output of the stomatogastric nervous system [38, 39]. However, their presence in the CG indicates that they may also perform some modulatory role within the cardiac neuromuscular system. Pyrokinin neuropeptides have also been mostly studied in the stomatogastric nervous system, but there has been at least one study demonstrating an excitatory role in the lobster cardiac neuromuscular system [8].
Conclusions
This study sought to identify and provide general localization information for neuropeptides within the crustacean cardiac ganglion, a small yet chemically complex neural circuit comprised of a small number of neurons. LC-MS/MS with a DIA method resulted in the identification of 316 neuropeptides, a surprisingly large number of peptides present in a small neuronal ganglion. After comparing MALDI-MSI platforms, a Bruker RapifleX TOF/TOF instrument was used to localize 235 neuropeptides to discrete regions of the CG with a high spatial resolution. Overall, the large number of neuropeptides being identified and localized within this tissue highlights the complexity of the modulatory circuits within the crustacean cardiac neuromuscular system, and the results presented here demonstrate the powerful capabilities of MALDI-MS imaging with a multifaceted approach for studying small neural networks. While the specific functions of these peptides within the CG are largely unknown, their presence within the tissue indicates that they may function as a molecular regulator in modulating heartbeat. Two important future directions will be to perform immunocytochemistry to obtain more detailed localization information and assess the function of these neuropeptides on cardiac output in both ex vivo and in vivo conditions in order to determine the biological activity of each neuropeptide.
Supplementary Material
Table S1. Neuropeptides localized in the CG with MALDI-MSI and the method used to confirm their identity is provided as supplemental material.
Acknowledgements
This work was supported by a National Science Foundation grant (CHE-1710140) and the National Institutes of Health (NIH) through grants 1R01DK071801 and R56 MH110215. The Orbitrap instruments were purchased through the support of an NIH shared instrument grant (NIH-NCRR S10RR029531). The MALDI TOF/TOF RapifleX mass spectrometer was purchased through the support of an NIH shared instrument grant S10OD025084. K.D. acknowledges a predoctoral fellowship supported by the National Institutes of Health-General Medical Sciences F31 National Research Service Award (1F31GM126870-01A1) for funding. LL acknowledges a Vilas Distinguished Achievement Professorship and Charles Melbourne Johnson Professorship with funding provided by the Wisconsin Alumni Research Foundation and University of Wisconsin-Madison School of Pharmacy.
Abbreviations:
- DHB
2,5 dihydroxybenzoic acid
- CG
cardiac ganglion
- CHH
crustacean hyperglycemic hormone
- CPRP
crustacean hyperglycemic hormone precursor related peptide
- CPS
Crystal positioning system
- DDA
data dependent acquisition
- DIA
data independent acquisition
- ESI
electrospray ionization
- FA
formic acid
- ITO
indium tin oxide
- LC
liquid chromatography
- MS
mass spectrometry
- MSI
mass spectrometry imaging
- MALDI
matrix-assisted laser desorption/ionization
- PO
pericardial organ
- TIC
total ion current
- MS/MS
tandem mass spectrometry
- TOF
time of flight
References
- 1.Marder E, Bucher D: Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 69, 291–316 (2007) [DOI] [PubMed] [Google Scholar]
- 2.DeLaney K, Buchberger AR, Atkinson L, Grunder S, Mousley A, Li L: New techniques, applications and perspectives in neuropeptide research. J Exp Biol. 221, (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuramoto T, Yamagishi H: Physiological anatomy, burst formation, and burst frequency of the cardiac ganglion of crustaceans. Physiological Zoology. 63, 102–116 (1990) [Google Scholar]
- 4.Ransdell JL, Temporal S, West NL, Leyrer ML, Schulz DJ: Characterization of inward currents and channels underlying burst activity in motoneurons of crab cardiac ganglion. J Neurophysiol. 110, 42–54 (2013) [DOI] [PubMed] [Google Scholar]
- 5.Cooke IM: Reliable, responsive pacemaking and pattern generation with minimal cell numbers: the crustacean cardiac ganglion. Biol Bull. 202, 108–136 (2002) [DOI] [PubMed] [Google Scholar]
- 6.Cruz-Bermudez ND, Marder E: Multiple modulators act on the cardiac ganglion of the crab, Cancer borealis. J Exp Biol. 210, 2873–2884 (2007) [DOI] [PubMed] [Google Scholar]
- 7.McMahon BR, Burnett LE: The Crustacean Open Circulatory System: A Reexamination. Physiological Zoology. 63, 35–71 (1990) [Google Scholar]
- 8.Dickinson PS, Sreekrishnan A, Kwiatkowski MA, Christie AE: Distinct or shared actions of peptide family isoforms: I. Peptide-specific actions of pyrokinins in the lobster cardiac neuromuscular system. (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson PS, Armstrong MK, Dickinson ES, Fernandez R, Miller A, Pong S, Powers BW, Pupo-Wiss A, Stanhope ME, Walsh PJ, Wiwatpanit T, Christie AE: Three members of a peptide family are differentially distributed and elicit differential state-dependent responses in a pattern generator-effector system. J Neurophysiol. 119, 1767–1781 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickinson PS, Samuel HM, Stemmler EA, Christie AE: SIFamide peptides modulate cardiac activity differently in two species of Cancer crab. Gen Comp Endocrinol. 282, 113204 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kushinsky D, Morozova EO, Marder E: In vivo effects of temperature on the heart and pyloric rhythms in the crab Cancer borealis. J Exp Biol. 222, (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickinson PS, Calkins A, Stevens JS: Related neuropeptides use different balances of unitary mechanisms to modulate the cardiac neuromuscular system in the American lobster, Homarus americanus. J Neurophysiol. 113, 856–870 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickinson PS, Kurland SC, Qu X, Parker BO, Sreekrishnan A, Kwiatkowski MA, Williams AH, Ysasi AB, Christie AE: Distinct or shared actions of peptide family isoforms: II. Multiple pyrokinins exert similar effects in the lobster stomatogastric nervous system. J Exp Biol. 218, 2905–2917 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nusbaum MP, Blitz DM, Marder E: Functional consequences of neuropeptide and small-molecule co-transmission. Nat Rev Neurosci. 18, 389–403 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye H, Hui LM, Kellersberger K, Li LJ: Mapping of Neuropeptides in the Crustacean Stomatogastric Nervous System by Imaging Mass Spectrometry. Journal of the American Society for Mass Spectrometry. 24, 134–147 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Q, OuYang C, Liang Z, Li L: Mass spectrometric characterization of the crustacean neuropeptidome. EuPA Open Proteomics. 3, 152–170 (2014) [Google Scholar]
- 17.Zhang Y, DeLaney K, Hui L, Wang J, Sturm RM, Li L: A Multifaceted Mass Spectrometric Method to Probe Feeding Related Neuropeptide Changes in Callinectes sapidus and Carcinus maenas. J Am Soc Mass Spectrom. 29, 948–960 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao Q, Wang Y, Chen B, Ma F, Hao L, Li G, Ouyang C, Li L: Visualization and Identification of Neurotransmitters in Crustacean Brain via Multifaceted Mass Spectrometric Approaches. ACS Chem Neurosci. 10, 1222–1229 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeLaney K, Li L: Data Independent Acquisition Mass Spectrometry Method for Improved Neuropeptidomic Coverage in Crustacean Neural Tissue Extracts. Anal Chem. 91, 5150–5158 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adusumilli R, Mallick P: Data Conversion with ProteoWizard msConvert. Methods Mol Biol. 1550, 339–368 (2017) [DOI] [PubMed] [Google Scholar]
- 21.Tsou CC, Avtonomov D, Larsen B, Tucholska M, Choi H, Gingras AC, Nesvizhskii AI: DIA-Umpire: comprehensive computational framework for data independent acquisition proteomics. Nat Methods. 12, 258–264 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma B, Zhang KZ, Hendrie C, Liang CZ, Li M, Doherty-Kirby A, Lajoie G: PEAKS: powerful software for peptide de novo sequencing by tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 17, 2337–2342 (2003) [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Xin L, Shan B, Chen W, Xie M, Yuen D, Zhang W, Zhang Z, Lajoie GA, Ma B: PEAKS DB: de novo sequencing assisted database search for sensitive and accurate peptide identification. Mol Cell Proteomics. 11, M111.010587 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robichaud G, Garrard KP, Barry JA, Muddiman DC: MSiReader: An Open-Source Interface to View and Analyze High Resolving Power MS Imaging Files on Matlab Platform. Journal of the American Society for Mass Spectrometry. 24, 718–721 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Buchberger AR, DeLaney K, Li Z, Li L: Multifaceted Mass Spectrometric Investigation of Neuropeptide Changes in Atlantic Blue Crab, Callinectes sapidus, in Response to Low pH Stress. J Proteome Res. 18, 2759–2770 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeLaney K, Li L: Capillary electrophoresis coupled to MALDI mass spectrometry imaging with large volume sample stacking injection for improved coverage of C. borealis neuropeptidome. Analyst. 145, 61–69 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadler WM, Waidelich D, Kerner A, Hanke S, Berg R, Trumpp A, Rösli C: MALDI versus ESI: The Impact of the Ion Source on Peptide Identification. (2017) [DOI] [PubMed] [Google Scholar]
- 28.Skiebe P, Schneider H: Allatostatin peptides in the crab stomatogastric nervous system: inhibition of the pyloric motor pattern and distribution of allatostatin-like immunoreactivity. Journal of Experimental Biology. 194, 195–208 (1994) [DOI] [PubMed] [Google Scholar]
- 29.Jorge-Rivera J, Marder E: Allatostatin decreases stomatogastric neuromuscular transmission in the crab Cancer borealis. J Exp Biol. 200, 2937–2946 (1997) [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Buchberger A, Muthuvel G, Li L: Expression and distribution of neuropeptides in the nervous system of the crab Carcinus maenas and their roles in environmental stress. Proteomics. (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung JS, Zmora N, Katayama H, Tsutsui N: Crustacean hyperglycemic hormone (CHH) neuropeptides family: Functions, titer, and binding to target tissues. General and Comparative Endocrinology. 166, 447–454 (2010) [DOI] [PubMed] [Google Scholar]
- 32.Hsu YW, Messinger DI, Chung JS, Webster SG, de la Iglesia HO, Christie AE: Members of the crustacean hyperglycemic hormone (CHH) peptide family are differentially distributed both between and within the neuroendocrine organs of Cancer crabs: implications for differential release and pleiotropic function. J Exp Biol. 209, 3241–3256 (2006) [DOI] [PubMed] [Google Scholar]
- 33.Dockray GJ: The expanding family of -RFamide peptides and their effects on feeding behaviour. Exp Physiol. 89, 229–235 (2004) [DOI] [PubMed] [Google Scholar]
- 34.Bechtold DA, Luckman SM: The role of RFamide peptides in feeding. J Endocrinol. 192, 3–15 (2007) [DOI] [PubMed] [Google Scholar]
- 35.Findeisen M, Rathmann D, Beck-Sickinger AG: RFamide Peptides: Structure, Function, Mechanisms and Pharmaceutical Potential. (2011) [Google Scholar]
- 36.Stevens JS, Cashman CR, Smith CM, Beale KM, Towle DW, Christie AE, Dickinson PS: The peptide hormone pQDLDHVFLRFamide (crustacean myosuppressin) modulates the Homarus americanus cardiac neuromuscular system at multiple sites. J Exp Biol. 212, 3961–3976 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickinson PS, Stevens JS, Rus S, Brennan HR, Goiney CC, Smith CM, Li L, Towle DW, Christie AE: Identification and cardiotropic actions of sulfakinin peptides in the American lobster Homarus americanus. J Exp Biol. 210, 2278–2289 (2007) [DOI] [PubMed] [Google Scholar]
- 38.Li LJ, Pulver SR, Kelley WP, Thirumalai V, Sweedler JV, Marder E: Orcokinin peptides in developing and adult crustacean stomatogastric nervous systems and pericardial organs. Journal of Comparative Neurology. 444, 227–244 (2002) [DOI] [PubMed] [Google Scholar]
- 39.Dickinson PS, Stemmler EA, Barton EE, Cashman CR, Gardner NP, Rus S, Brennan HR, McClintock TS, Christie AE: Molecular, mass spectral, and physiological analyses of orcokinins and orcokinin precursor-related peptides in the lobster Homarus americanus and the crayfish Procambarus clarkii. Peptides. 30, 297–317 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christie AE, Cashman CR, Brennan HR, Ma MM, Sousa GL, Li LJ, Stemmler EA, Dickinson PS: Identification of putative crustacean neuropeptides using in silico analyses of publicly accessible expressed sequence tags. General and Comparative Endocrinology. 156, 246–264 (2008) [DOI] [PubMed] [Google Scholar]
- 41.Ma MM, Bors EK, Dickinson ES, Kwiatkowski MA, Sousa GL, Henry RP, Smith CM, Towle DW, Christie AE, Li LJ: Characterization of the Carcinus maenas neuropeptidome by mass spectrometry and functional genomics. General and Comparative Endocrinology. 161, 320–334 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma MM, Chen RB, Sousa GL, Bors EK, Kwiatkowski MA, Goiney CC, Goy MF, Christie AE, Li LJ: Mass spectral characterization of peptide transmitters/hormones in the nervous system and neuroendocrine organs of the American lobster Homarus americanus. General and Comparative Endocrinology. 156, 395–409 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Neuropeptides localized in the CG with MALDI-MSI and the method used to confirm their identity is provided as supplemental material.