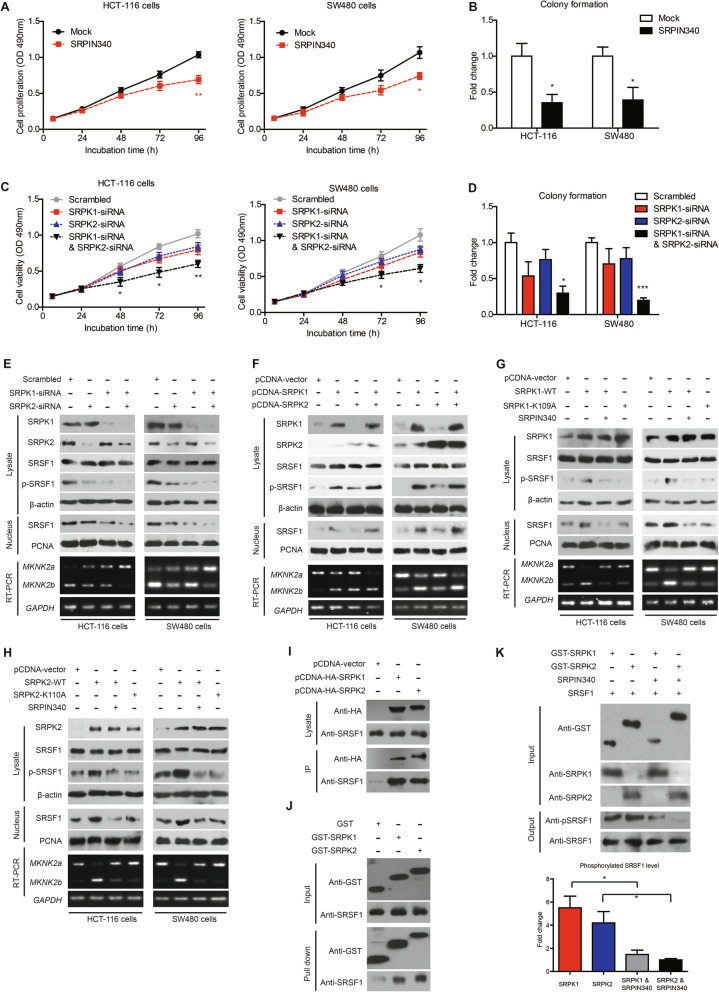
Fig. 5.
SRPKs promote SRSF1 phosphorylation, nucleus transportation, and downstream MKNK2 alternative splicing. a Treatment with SRPKs inhibitor, SRPIN340, resulted in decreased proliferation of HCT-116 and SW480 cells. P value was based on unpaired Student’s t-test comparing with mock group which was treated with DMSO. b Colony formation assay also demonstrated an anti-proliferation effect of SRPIN340 on CAC cells, indicating the oncogenic role of SRPKs. P value was based on unpaired Student’s t-test comparing with mock group. c MTT and colony formation (d) assays demonstrated that silencing either SRPK1 or SRPK2 may impair CAC proliferation, although the statistical difference was not significant. Knockdown of both SRPK1 and SRPK2 can significantly inhibit CAC cell proliferation. P value was based on unpaired Student’s t-test comparing with scrambled group. e Knockdown of either SRPK1 or SRPK2 attenuated phosphorylation of SRSF1 (p-SRSF1) and reduced nucleus SRSF1 (N-SRSF1) level. RT-PCR results showed a positive correlation between MKNK2b and SRPKs. Furthermore, simultaneously knockdown of SRPK1 and SRPK2 exhibited the most significant effects. Semi-quantification of immunoblotting results and corresponding RT-qPCR data were shown in Supplementary Figure S7A-B. f Overexpressing SRPKs increased phosphorylation of SRSF1 and promoted its nucleus translocation according to immunoblotting results. Semi-quantification of immunoblotting results and corresponding RT-qPCR data were shown in Supplementary Figure S7C-D. g SRPIN340 treatment towards SRPK1-overexpressing cells abolished the effects above. Consistently, the kinase dead (KD) mutant SRPK1-K109A, lost effects on modulating SRSF1 phosphorylation, subcellular location, and MKNK2a-MKNK2b switch. Semi-quantification of immunoblotting results and corresponding RT-qPCR data were shown in Supplementary Figure S7E-F. h SRPIN340 treatment towards SRPK2-overexpressing cells also abolished its effects on modulating SRSF1 phosphorylation, subcellular location, and MKNK2a-MKNK2b switch. The KD mutant of SRPK2, namely SRPK2-K110A, showed similar effects with SRPIN340. Semi-quantification of immunoblotting results and corresponding RT-qPCR data were shown in Supplementary Figure S7G-H. i SW480 cells were transfected with HA-tagged SRPK1 (pcDNA-SRPK1) or HA-tagged SRPK2 (pcDNA-SRPK2) or pcDNA-vector constructs. After lysing cells and conducting immunoprecipitation assay with anti-HA agarose, we found that SRPK1 and SRPK2 can interact with SRSF1 in CAC cells. j Purified GST-tagged SRPK1 or GST-SRPK2 proteins were incubated with purified SRSF1 proteins in vitro using GST protein as negative control. GST pull-down assay demonstrated the direct interaction between SRPK1/2 with SRSF1. The input was shown in upper panel. k The kinase activity of SRPK1/2 targeting SRSF1 was tested by in vitro kinase assay. Purified SRSF1 was incubated with GST-SRPK1 or GST-SRPK2 with/without SRPIN340 (10 μM) as described in the Method section. Reaction products were separated via SDS-PAGE and analyzed by western blotting. Phosphorylation of SRSF1 in reaction products was semi-quantified after normalized by total SRSF1 (lower panel). The data showed that both SRPK1 and SRPK2 can directly phosphorylate SRSF1, which can be inhibited by the inhibitor SRPIN340