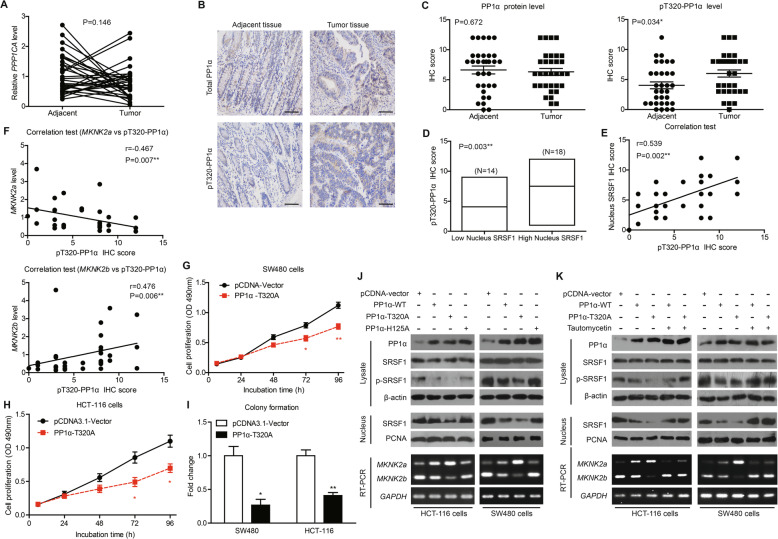
Fig. 6.
PP1α activity is decreased in CAC tissues and correlates with SRSF1-dependent MKNK2 alternative splicing. a There was no statistical difference of PPP1CA mRNA levels between tumor tissues and adjacent nontumorous tissues. P value was based on paired Student’s t-test (n = 32). b Representative IHC images showed the immunoreactivities of total PP1α and phosphorylated-PP1α (pT320-PP1α) in clinical specimens. Scale bar: 100 μm. c Although no difference regarding total PP1α protein level was observed between tumor and adjacent tissues (left panel), tumor tissues exhibited higher phosphorylation on T320 site (pT320-PP1α, right panel) based on IHC data. P value was based on paired Student’s t-test (n = 32). d Tumor tissues with higher nucleus SRSF1 showed higher pT320-PP1α levels. P value was based on unpaired Student’s t-test. e Spearman correlation test further confirmed a positive correlation between nucleus SRSF1 and pT320-PP1α levels. f Spearman correlation test identified a negative correlation between pT320-PP1α and MKNK2a levels. In contrast, MKNK2b was positively correlated with pT320-PP1α level. g Proliferation capacity of SW480 cells or HCT-116 cells (h) was impaired by overexpressing pcDNA-PP1α-T320A, a constitutively active PP1α construct, as revealed by MTT assays. P value was based on unpaired Student’s t-test. i Colony formation assay was conducted for the cells described in (g) and (h), further indicating that PP1α-T320A may attenuate CAC proliferation. j CAC cells were transfected with three different PP1α constructs, including PP1α-WT, PP1α-T320A (constitutively active mutant), and PP1α-H125A (kinase-dead mutant) using pcDNA-vector as control. The levels of phosphorylated SRSF1, nucleus SRSF1 were measured by western blotting, and the MKNK2 splicing were evaluated by RT-PCR (lower panel). Semi-quantification of immunoblotting results and corresponding RT-qPCR data were shown in Supplementary Figure S8D. (K) CAC cells were transfected with PP1α-WT or PP1α-T320A with/without tautomycetin treatment. The levels of phosphorylated SRSF1, nucleus SRSF1 were measured by western blotting, and MKNK2 splicing were evaluated by RT-PCR (lower panel). Semi-quantification of immunoblotting results and corresponding RT-qPCR data were shown in Supplementary Figure S8E