Figure 2.
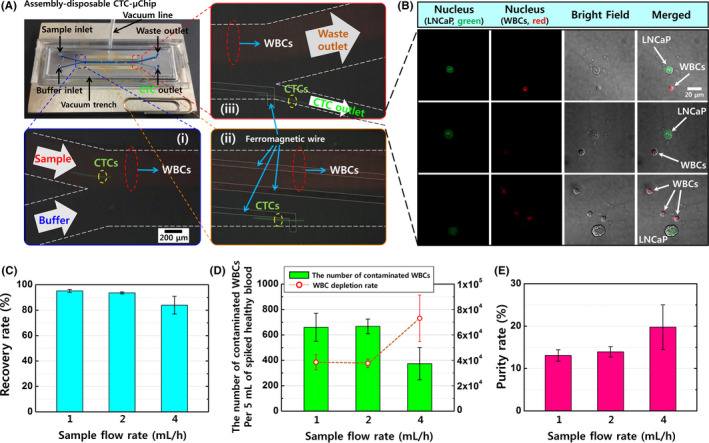
A, The lateral magnetophoretic microseparator and its experimental set‐up, including two stacked neodymium‐iron‐boron permanent magnets and a vacuum tube applying −50 kPa of pressure to assemble the disposable polymeric superstrate and the reusable substrate. Magnified views show the three spots in the microchannel where the circulating tumor cells (CTCs) are separated from the blood by the lateral magnetic force and flow into the CTC outlet. (i) A magnified view of the sample and buffer injection channel, with the blood sample entering the upper channel because of laminar flow. (ii) The middle region of the microchannel allows for lateral isolation of the CTCs by the ferromagnetic wires. (iii) The CTC isolation is completed via the CTC outlet, while most white blood cells (WBCs) continue to flow through the waste outlet. B, Immunofluorescent images of the retrieved LNCaP cells and coisolated WBCs, which were stained using SYTO 13 dye (green) for LNCaP cells and SYTO 64 dye (red) for nucleated cells (LNCaP cells and WBCs). C, The recovery rates for LNCaP cells from spiked healthy blood samples using the lateral magnetophoretic microseparator and various flow rates. D, The numbers of contaminated WBCs and the WBC depletion rates. E, The purity rates for LNCaP cells. Approximately 100 LNCaP cells were spiked into 5 mL of peripheral blood from a healthy donor and tagged using anti‐EpCAM*‐based magnetic nanobeads, with retrieval performed at sample and buffer flow rates of 1 mL/h, 2 mL/h, and 4 mL/h (external magnetic flux: 0.2 T). Error bars represent the standard deviation for three measured datasets
