Abstract
Near‐infrared photoimmunotherapy (NIR‐PIT) is a novel therapy for cancers that uses NIR light and antibody‐photosensitizer (IR700) conjugates. However, it is difficult to deliver NIR light into the bile duct for cholangiocarcinoma (CCA) from the conventional extracorporeal apparatus. Thus, in this study, we developed a dedicated catheter with light emitting diodes (LEDs) that supersedes conventional external irradiation devices; we investigated the therapeutic effect of NIR‐PIT for CCA using the novel catheter. The new catheter was designed to be placed in the bile duct and a temperature sensor was attached to the tip of the catheter to avoid thermal burn. An anti‐epidermal growth factor receptor (EGFR) antibody, Panitumumab‐IR700 conjugate or anti‐human epidermal growth factor receptor type 2 (HER2) antibody, Trastuzumab‐IR700 conjugate, was used with EGFR‐ or HER2‐expressing cell lines, respectively. The in vitro efficacy of NIR‐PIT was confirmed in cultured cells; the capability of the new catheter for NIR‐PIT was then tested in a mouse tumor model. NIR‐PIT via the developed catheter treated CCA xenografts in mice. NIR‐PIT had an effect in Panitumumab‐IR700 conjugate‐ and Trastuzumab‐IR700 conjugate‐treated CCA cells that depended on the receptor expression level. Tumor growth was significantly suppressed in mice treated with NIR‐PIT using the novel catheter compared with controls (P < .01). NIR‐PIT was an effective treatment for EGFR‐ and HER2‐expressing CCA cells, and the novel catheter with mounted LEDs was useful for NIR‐PIT of CCA.
Keywords: catheter, cholangiocarcinoma, light emitting diode, near‐infrared photoimmunotherapy
A new catheter with light emitting diodes for photoimmunotherapy for cholangiocarcinoma was developed. The new catheter was designed to be placed in the bile duct and a temperature sensor was attached to the tip of the catheter to avoid thermal burn. Tumor growth was significantly suppressed in mice treated with NIR‐PIT using the novel catheter.
1. INTRODUCTION
Cholangiocarcinoma (CCA) is relatively rare and accounts for about 3% of all gastrointestinal cancers in the United States and 5% in Japan. The overall 5‐y survival rate of CCA is 20%‐30% with a poor prognosis secondary to only to pancreatic cancer. 1 , 2 The reason for this low survival rate is the limited CCA treatment methods available. For example, surgical resection or liver transplantation is performed as a radical treatment for CCA, and palliative chemotherapy alone with gemcitabine and cisplatin is used for patients with advanced disease. 3 Although conventional chemotherapies have been used in the clinic for patients with advanced disease, previous reports have demonstrated that CCA cells express therapeutic targets such as epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor type 2 (HER2). ErbB receptors (including EGFR and HER2) on normal bile duct epithelial cells are considered to play a role in cholangiocyte specification and bile duct morphogenesis. 4 It has been reported that EGFR is overexpressed by 11%‐27% in intrahepatic CCA and by 5%‐19% in extrahepatic CCA, and HER2 is overexpressed by 10%‐15% in extrahepatic CCA and gallbladder carcinoma, whereas normal bile duct epithelial cells do not overexpress these receptors. 5 , 6 These reports also indicated the potential of performing novel molecular targeted therapy. However, chemotherapies targeting EGFR and HER2 have failed because of insufficient therapeutic effects compared with conventional therapies. 7 , 8 Therefore, in clinical practice a novel approach to treat CCA other than surgery or chemotherapy is desperately needed.
Near‐infrared photoimmunotherapy (NIR‐PIT) is an evolutional therapy for various cancers that uses NIR light and monoclonal antibody‐photosensitizer conjugates. 9 When the conjugates bind to the membrane of the target cells and are exposed to NIR light, immunogenic cancer cell death is induced without damaging the adjacent normal cells. 9 , 10 , 11 , 12 , 13 A clinical trial of NIR‐PIT for patients with recurrent head and neck cancer that could not be cured with chemotherapy, radiation therapy, or surgery, was approved by the US Food and Drug Administration in 2015. A phase III trial is now ongoing for patients who have failed at least 2 lines of conventional chemotherapies (https://clinicaltrials.gov/ct2/show/NCT03769506). NIR‐PIT might be useful to treat CCA in patients who have not responded to antibodies or standard chemotherapies because it is a novel molecular targeted therapy.
To date, laser light has been the primary light source used in conventional clinical photodynamic therapy. 14 An ongoing clinical trial of NIR‐PIT uses a light diffuser attached to the tip of a laser inserted into the tumor from the skin to deliver sufficient amounts of NIR light. An endoscopic approach might be more effective at delivering NIR by light diffuser into deep regions such as the gastrointestinal tract or urinary bladder. 15 , 16 Furthermore, delivery of NIR laser by light diffuser into deep lesions in retroperitoneal organs such as the bile duct, pancreas, urinary tract, and spleen is difficult when using an endoscope because implanting a light diffuser in these organs is highly invasive and it is difficult to repeatedly irradiate tissues with a continuous indwelling. Furthermore, there is a risk of kinking during deep insertion when using an optical fiber diffuser. However, a catheter with a flexible printed circuit board on which LEDs can be mounted, has flexibility and non‐kinking properties, allowing for deep insertion and irradiation with endoscopes and guidewires. Therefore, there is a need to develop a new light‐irradiation flexible catheter that can be safely implanted long term using assisting devices.
The aim was to treat intractable CCA by NIR‐PIT with a newly developed catheter device with LEDs that can be inserted into the bile duct and emit NIR light to the tumor.
2. MATERIALS AND METHODS
2.1. Development of a novel wired NIR‐LED catheter
We newly developed an NIR‐LED catheter that has 2 lumens, one dedicated to LED insertion and the other for access of devices such as a guidewire (Figure 1). The outer diameter of the catheter made of polyurethane was 2.2 mm. LEDs (SMT690D; USHIO OPTO SEMICONDUCTORS INC.) that emit light of 680‐700 nm wavelength were evenly spaced at 3‐mm intervals on a flexible printed circuit (FPC) with a longitudinal length of 20.0 mm. Power density was measured with an optical power meter (PM121D; Thorlabs, 20 mW/cm2). Two types of K thermocouples were attached to the FPC board, one located directly under the LED to measure its temperature and the other located at the distal end of the FPC to measure the temperature out of the catheter. The LED temperature was monitored by a control device. When the temperature exceeded the set temperature of 50°C, the LEDs turned off automatically.
FIGURE 1.
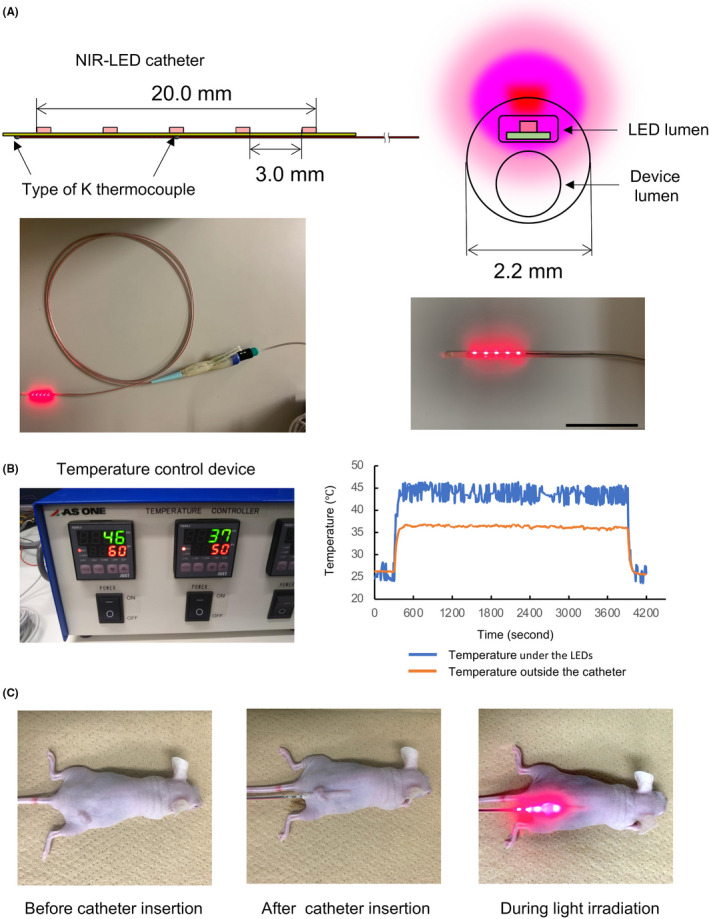
NIR‐LEDs catheter. A, Schema of the LEDs and flexible printed circuits. Five 1.6 mm LED chips were mounted on the catheter (upper left). A cross‐section of a catheter with an LED lumen and device lumen. The catheter could generate NIR light from the upper side of the LED lumen (upper right). Overview of the wired NIR‐LED catheter (lower left). The front of the catheter (lower right). Scale bar: 30 mm. B, The temperature control device (left). Measurement results of the catheter temperature under the LEDs and outside the catheter during LED lights on (right). C, The NIR‐LED catheter device was subcutaneously inserted into a tumor‐bearing mouse. The tumor was irradiated with the NIR‐LED catheter device from a site below the tumor
2.2. Antibodies and reagents
Panitumumab (Vectibix®), a fully humanized IgG2 monoclonal antibody (mAb) directed against EGFR, was purchased from Takeda Pharmaceutical Co. Ltd. Trastuzumab (Herceptin®), a 95% humanized IgG1 mAb directed against human HER2, was purchased from Chugai Pharmaceutical Co., Ltd. A water‐soluble silicon‐phthalocyanine derivative, IRDye 700DX (IR700) NHS ester was obtained from Li‐COR Bioscience. All other chemicals used were of reagent grade.
2.3. Synthesis of IR700‐conjugated antibodies
The methods of conjugation were referenced in a previous report.9 Briefly, panitumumab or trastuzumab (1 mg, 6.8 nmol) were incubated with IR700 NHS ester (66.8 µg, 34.2 nmol) in 0.1 M Na2HPO4 (pH 8.5) at room temperature for 3 h. The mixture was purified with a gel‐filtration Sephadex G50 column (PD‐10; GE Healthcare), and Panitumumab‐IR700 (Pan‐IR700) or Trastuzumab‐IR700 (Tra‐IR700) conjugates were derived. Protein concentrations were determined using a Modified Lowry Protein Assay Kit (Thermo Fisher Scientific Inc) by measuring the absorption at 750 nm with a microplate reader (Infinite M200; Tecan Austria GmbH). The concentration of IR700 was measured by absorption at 689 nm with a spectrophotometer (UV‐1800; Shimadzu Corp.) to confirm the number of fluorophore molecules to each mAb. The IR700 to mAb ratio was approximately 3.
2.4. Cell lines and cell culture
Three human CCA cell lines, EGFR‐expressing HuCCT‐1 and YSCCC lines, and the HER2‐expressing TFK‐1 line were obtained from the RIKEN BioResource Research Center Cell Bank (Ibaraki, Japan). The combined hepatocellular‐CCA cell line, HER2 expressing KMCH‐1 was provided by Dr. H. Yano (Kurume University School of Medicine, Kurume, Japan). All cell lines were grown in RPMI‐1640 medium (Sigma) containing 10% fetal bovine serum (Gibco Life Technologies) and 1% penicillin/streptomycin (Nacalai Tesque Inc) in tissue culture dishes in a humidified incubator at 37°C in an atmosphere composed of 95% air and 5% carbon dioxide.
2.5. Fluorescence microscopic observation of the binding of IR700 conjugates
HuCCT‐1, YSCCC, TFK‐1, and KMCH‐1 cells were seeded onto 35‐mm culture dishes and incubated for 24 h at 37°C. Pan‐IR700 or Tra‐IR700 was then added to the culture medium at 5 µg/mL and incubated for 1 h at 37°C. After washing with phosphate buffered saline (PBS), the cells were observed using a fluorescence microscope (CKX41; Olympus Corporation). Fluorescence microscopy was performed using combinations of 673‐748 nm excitation filter and 765‐855 nm emission filters for IR700. Image analysis was conducted using ImageJ software (http://rsb.info.nih.gov/ij/).
2.6. Flow cytometry
Flow cytometry analysis was performed to investigate the EGFR and HER2 expressions of HuCCT‐1, YSCCC, TFK‐1, and KMCH‐1 cells. The cells were seeded on 35‐mm culture dishes and incubated for 24 h at 37°C. Panitumumab or Trastuzumab labeled with Alexa Fluor 488 (Thermo Fisher Scientific Inc) and Panitumumab‐Alexa488 (Pan‐Alexa488) or Trastuzumab‐Alexa488 (Tra‐Alexa488) conjugates were then derived. Pan‐Alexa488 or Tra‐Alexa488 was added to the culture medium at 10 µg/mL and cells incubated for 6 h at 37°C. After washing with PBS, cells were harvested, fluorescence intensity was then measured using a flow cytometer (Gallios; Beckman Coulter, Inc). To evaluate the specific binding of antibody conjugates, an excess dose of unconjugated antibody (100 µg/mL) was added to block binding of conjugates to cells. Data were analyzed using Kaluza Analysis 2.1 software (Beckman Coulter).
2.7. In vitro NIR‐PIT: fluorescence microscopy
Calcein AM/Ethidium homodimer‐1 (EthD‐1) (Thermo Fisher Scientific Inc) was used to detect live and dead cells after NIR‐PIT (LIVE/DEAD staining). The cells were irradiated with NIR light using an LED for 60 s (3 J/cm2). Power density was measured with an optical power meter (50 mW/cm2), cells were then observed using a fluorescence microscope at 1 h after the onset of light exposure. Next, HuCCT‐1 and KMCH‐1 cells were seeded onto 60‐mm culture dishes and incubated for 24 h at 37°C. Pan‐IR700 or Tra‐IR700 was then added to the culture medium at 5 µg/mL and incubated for 1 h at 37°C; cells were then irradiated with NIR light using the LED catheter in the culture medium for 500 s (10 J/cm2). Cells were observed using a fluorescence microscope at 1 h after the onset of light exposure. The capability of the LED catheter to immerse the tip in culture medium and the possibility to continuously irradiate light during treatment was then confirmed. Fluorescence microscopy was performed using combinations of 460‐490 nm excitation filters and a 520 nm long‐pass emission filter for calcein, or combinations of 480‐550 nm excitation filters and a 590 nm long‐pass emission filter for EthD‐1.
2.8. In vitro NIR‐PIT: flow cytometry
CCA cells (5 × 105) were seeded on 35‐mm culture dishes and incubated for 24 h at 37°C. Pan‐IR700 at 10 µg/mL was added to culture medium containing HuCCT‐1 and YSCCC cells. Tra‐IR700 at 10 µg/mL was added to the culture medium containing TFK‐1 and KMCH‐1 cells. After incubation for 6 h at 37°C, the cells were irradiated with NIR light using an LED at a dose of 1, 2, 5, 10, or 30 J/cm2; 1 h later, irradiated cells were harvested and suspended in PBS. Propidium iodide (Life Technologies), which detects dead cells, was added to the suspension and cells were incubated for 30 min at room temperature. After NIR‐PIT, flow cytometry analysis was then performed to detect the propidium iodide‐stained cells. Data were analyzed using Kaluza Analysis 2.1 software (Beckman Coulter).
2.9. Animal and tumor model
All in vivo experimental works were performed with approval from the Hokkaido University Animal Care Committee in accordance with the guidelines for the care and use of laboratory animals. BALB/c Slc‐nu/nu nude mice (6 wk old females) were purchased from Japan SLC, Inc. During the following procedures, mice were anesthetized with isoflurane.
Four million HuCCT‐1 cells suspended in 100 µL of PBS were injected subcutaneously in the right side of the dorsum. Treatment was started after the tumor had reached a volume of 150 mm3 following tumor cell injection. To measure the tumor volume, the greatest longitudinal diameter (length) and the greatest transversal diameter (width) of tumors were measured with a caliper. Tumor volume was calculated using the following equation: volume = length × width2 × 0.5. 17 Mice were observed carefully for skin damage after treatment.
2.10. In vivo NIR‐PIT
Thirty‐two mice with a HuCCT‐1 tumor on the right dorsum were randomized into 8 groups to evaluate the effect of NIR‐PIT using the NIR‐LED catheter device: (group 1) 100 µg of Pan‐IR700 intravenous injection (iv) on day 0, with insertion of the NIR‐LED catheter under the tumor and irradiated with NIR light for 1 h (72 J/cm2) on day 1; (group 2) 100 µg of Pan‐IR700 iv on day 0, with insertion of a dummy LED catheter under the tumor (not emitting NIR light) on day 1; (group 3) 100 µg of Pan‐IR700 iv on day 0 only (without device); (group 4) no iv injection on day 0, with insertion of the NIR‐LED catheter under the tumor and irradiated with NIR light for 1 h (72 J/cm2) on day 1; (group 5) no iv injection on day 0, with insertion of a dummy LED catheter under the tumor (not emitting NIR light) on day 1; (group 6) 100 µg of Pan‐IR700 intravenous injection (iv) on day 0, irradiated with external NIR laser light (MLL‐III‐690‐800 mW; Changchun New Industries Optoelectronics Tech, Co., Ltd.) for 288 s (250 mW/cm2, 72 J/cm2) on day 1; (group 7) 100 µg of Tra‐IR700 intravenous injection (iv) on day 0, with insertion of the NIR‐LEDs catheter under the tumor and irradiated with NIR light for 1 h (72 J/cm2) on day 1; and (group 8) no treatment (no iv injection without device). After treatment, the tumor volume and body weight of individual mice were measured 3 times a week for 2 wk. IR700 fluorescence imaging of 32 mice was conducted on day 1 (before and immediately after NIR light irradiation), and day 2 using the FluorVivo Imaging System (Indec BioSystem) (excitation: 600‐640 nm, emission: 665 nm long pass). Fluorescence image analysis was performed using ImageJ software.
2.11. Histological analysis
To evaluate any histological changes in the tumor after NIR‐PIT with the NIR‐LED catheter device, 1 mouse from group 1 [NIR(+) Pan‐IR700(+) device(+)] and 1 mouse from group 8 [NIR(−) Pan‐IR700(−) device(−)] were euthanized on day 7. Extracted tumors were frozen in an optimal cutting temperature compound. Frozen slice sections (10 µm thickness) were prepared and stained with hematoxylin and eosin (H&E) in accordance with standard protocols. The slides were imaged with an Olympus BX41 microscope (Olympus Corporation) with 4× and 20× objective lenses.
2.12. Statistical analysis
Measured quantities were expressed as means ± SEM (standard error of the mean). Statistical analyses were carried out using JMP Pro 14.0.0 software (SAS Institute Inc). One‐way ANOVA and Dunnett's test were used for multiple comparisons. P values < .05 were considered to be statistically significant.
3. RESULTS
3.1. Development of a novel wired NIR‐LED catheter
Although our catheter was thin (diameter of 2.2 mm), it had double lumens for LED insertion and access of devices such as a guidewire. Given its potential use in clinical situations, this size is sufficient to allow its insertion through the forceps mouth of an endoscope. In addition, this catheter was soft enough for insertion and could not be broken by bending because of the small size of LEDs on the FPC. The wired NIR‐LED catheter was equipped with 5 LED sources in a 20‐mm‐long space, and the irradiation area was sufficiently wide to cover a mouse tumor (Figure 1A). Furthermore, the catheter had a thermocouple thermometer for temperature measurement, which enabled us to perform NIR‐PIT while checking the catheter temperature to avoid burn injury. During NIR light emission from this catheter, the temperature near the LED was approximately 46°C and the temperature outside the catheter was 37°C when measured in a room environment (Figure 1B). Therefore, thermal burns and the automatically turning off of LEDs did not occur during irradiation, because the site of the catheter in contact with body was under 37°C.
3.2. Expression of EGFR and HER2 by CCA cell lines
To investigate the usage of the newly developed LED catheter for NIR‐PIT, in vitro basic experiments using CCA cells were performed. In fluorescence imaging with microscopy demonstrated IR700 fluorescence in HuCCT‐1 and YSCCC, indicating that these cell lines expressed EGFR on their cell surface (Figure 2A). IR700 fluorescence was also observed in KMCH‐1, indicating that this cell expresses HER2 on the cell surface. These results suggested that Pan‐IR700 binds to EGFR on HuCCT‐1 and YSCCC cells, and that Tra‐IR700 binds to HER2 on KMCH‐1 cells.
FIGURE 2.
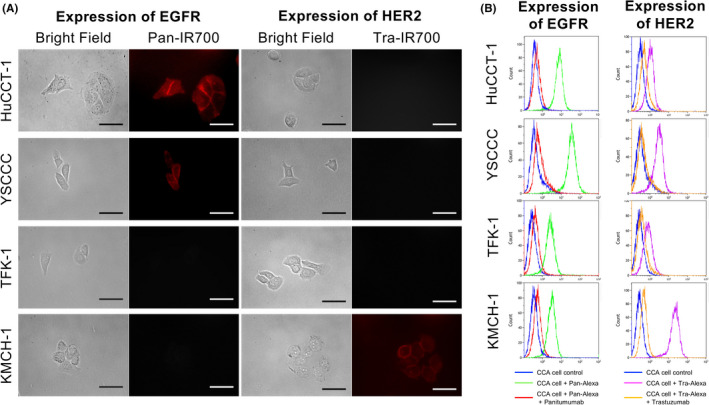
In vitro expression of EGFR and HER2 on CCA cells. A, Fluorescence microscopic images of HUCCT‐1, YSCCC, TFK‐1, and KMCH‐1 cells. All cells were pre‐incubated with Pan‐IR700 (5 µg/mL) or Tra‐IR700 (5 µg/mL) at 37°C for 1 h. Cells were then washed with PBS and IR700 fluorescence was observed. Scale bar: 50 µm (original magnification, 40×). B, Expression levels of EGFR and HER2 in HuCCT‐1, YSCCC, TFK‐1, and KMCH‐1 cells were evaluated by flow cytometry. Binding of Pan‐Alexa488 to HuCCT‐1 and YSCCC cells, and Tra‐Alexa488 to KMCH‐1 cells was blocked by adding an excess dose of unconjugated antibodies
Flow cytometry assay was used for the quantitative analysis of EGFR or HER2 expression, which was consistent with the results of fluorescence imaging. Binding of the corresponding Pan‐Alexa488 reagent was observed in HuCCT‐1 and YSCCC cells, and Tra‐Alexa‐488 was observed in KMCH‐1 cells, but not in TFK‐1 cells (Figure 2B). Binding of Pan‐Alexa488 to HuCCT‐1 and YSCCC cells and binding of Tra‐Alexa488 to KMCH‐1 cells were inhibited by adding an excess dose of the corresponding unconjugated antibodies. These results suggested that Pan‐Alexa488 specifically bound to EGFR on HuCCT‐1 and YSCCC cells, and that Tra‐Alexa488 specifically bound to HER2 on KMCH‐1 cells. In summary, fluorescence microscopy and flow cytometry data indicated that HuCCT‐1 and YSCCC cells expressed EGFR and that KMCH‐1 cells expressed HER2.
3.3. In vitro NIR‐PIT for CCA cells
Figure 3A shows the microscopy images of cells treated with NIR‐PIT for EGFR‐expressing HuCCT‐1, and YSCCC cells, and HER2‐expressing KMCH‐1 and TFK‐1 cells. LIVE/DEAD staining of HuCCT‐1, YSCCC, and KMCH‐1 cells showed that calcein was released and EthD‐1 entered these cell lines after light irradiation. Cell dysfunction such as swelling and bleb formation, hallmarks of necrotic cell death, were observed in these 3 cell lines under bright field imaging. In contrast, no apparent changes were observed after NIR light irradiation in TFK‐1, which lacked HER2 expression (Figure 3A). In NIR‐PIT with the LED catheter, EthD‐1 was found to enter HuCCT‐1 and KMCH‐1 cells and cell death could be confirmed in both cells at 1 h after NIR light irradiation (Figure 3B). We could confirm the capability of the LED catheter to perform continuous NIR irradiation with the tip place in the culture medium, and the same therapeutic effect on CCA cells compared with external irradiation.
FIGURE 3.
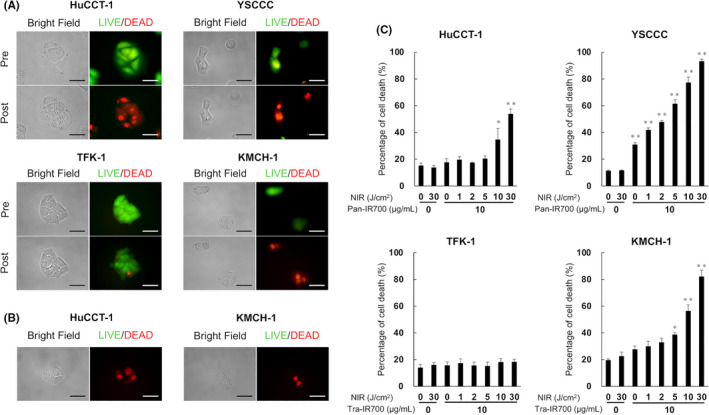
In vitro NIR‐PIT for CCA cells. A, LIVE/DEAD staining of NIR‐PIT‐treated CCA cells. HuCCT‐1 and YSCCC cells were pre‐incubated with Pan‐IR700 (5 µg/mL) at 37°C for 1 h. TFK‐1 and KMCH‐1 cells were pre‐incubated with Tra‐IR700 (5 µg/mL) at 37°C for 1 h. After NIR light irradiation, cell swelling, and bleb formation were observed in HuCCT‐1, YSCCC, and KMCH‐1 cells. The significant uptake of EthD‐1 (red) and leakage of calcein (green) in these cells was also detected at post NIR‐PIT. Scale bar: 50 µm (original magnification, 40×). B, LIVE/DEAD staining of NIR‐PIT‐treated HuCCT‐1 and KMCH‐1 cells with NIR‐LED catheter in culture fluid. HuCCT‐1 cells were pre‐incubated with Pan‐IR700 (5 µg/mL) at 37°C for 1 h. KMCH‐1 cells were pre‐incubated with Tra‐IR700 (5 µg/mL) at 37°C for 1 h. The significant uptake of EthD‐1 (red) in these cells was also detected at post NIR‐PIT. Scale bar: 50 µm (original magnification, ×40). C, Quantification of cell death induced by NIR‐PIT. Flow cytometry analysis with propidium iodide on CCA cells after NIR‐PIT. Increased percentages of death for HuCCT‐1, YSCCC, and KMCH‐1 cells were NIR light‐dose dependent. TFK‐1 was not killed by adding Tra‐IR700 and irradiating with NIR light. Data are presented as the means ± SEM (n = 4, *P < .05, **P < .01, vs untreated control, Dunnett's test)
Quantitative analysis of cell death by flow cytometry showed that the percentage of cell death for both EGFR‐expressing HuCCT‐1 and YSCCC cells was increased in a light‐dose‐dependent manner (Figure 3C). A similar tendency was also observed in HER2‐expressing KMCH‐1 cells. Taken together, these results indicated that NIR‐PIT induced death in CCA cells that expressed EGFR or HER2 as a target antigen.
3.4. In vivo NIR‐PIT with a novel wired NIR‐LED catheter
To evaluate the efficacy of the newly developed LED catheter for NIR‐PIT, in vivo experiments using HuCCT‐1 tumor‐bearing mice were performed. The catheter was inserted subcutaneously under the tumor, which was irradiated with NIR light, as shown in Figure 1C. Treatment and imaging were performed as shown in Figure 4A. IR700 fluorescence imaging demonstrated Pan‐IR700 accumulation in the tumors of group 1 mice [NIR(+) Pan‐IR700(+) device(+)] before NIR light irradiation on day 1 (Figure 4B). The fluorescence signal from IR700 clearly disappeared after light irradiation, suggesting that the tumor was effectively exposed to NIR light when using the NIR‐LED catheter. 18 On day 2, tumors from group 1 mice observed by fluorescence imaging indicated that Pan‐IR700 had re‐accumulated in the light‐irradiated tumor. A similar trend was also observed in external irradiation group 6 [NIR(+) Pan‐IR700(+) external irradiation(+)]. In contrast, Pan‐IR700 accumulation was continuous in all tumors from group 2 mice [NIR(−) Pan‐IR700(+) device(+)], group 3 [NIR(−) Pan‐IR700(+) device(−)] until day 2 (Figure 4B). Tra‐IR700 accumulation was not observed in the tumors from group 7 mice [NIR(+) Tra‐IR700(+) device(+)].
FIGURE 4.
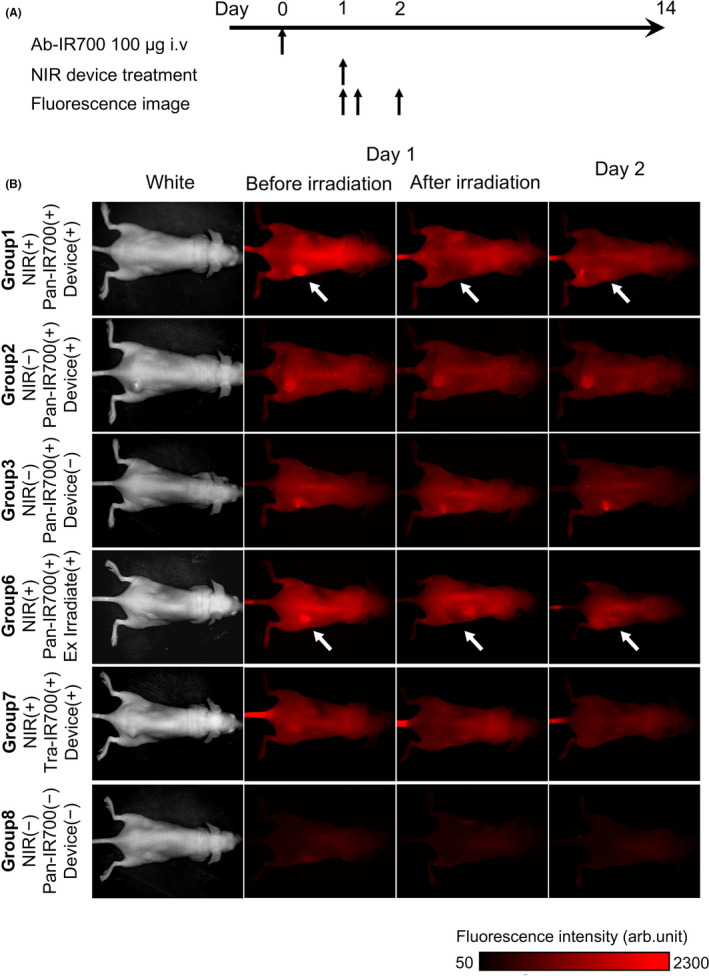
In vivo NIR‐PIT in mice: protocol and fluorescence imaging. A, Protocol of in vivo NIR‐PIT. Fluorescence imaging was performed before and immediately after NIR light irradiation on days 1, and 2. B, Fluorescence imaging of the 8 mouse groups. In group 1 [NIR(+) IR700(+) Device(+)], and group 6 mice [NIR(+) Pan‐IR700(+) external irradiation(+)] IR700 fluorescence was detected before NIR‐PIT, and disappeared immediately after treatment. IR700 fluorescence was observed in the tumors of group 1 mice on day 2 (white arrows). However, in group 2 and group 3 mice, IR700 fluorescence was continuously observed during this treatment. In group 7 mice, IR700 fluorescence in the tumor was not observed during treatment
HuCCT‐1 tumor growth in group 1 mice [NIR(+) Pan‐IR700(+) device(+)] and group 6 mice [NIR(+) Pan‐IR700(+) external irradiation(+)] was significantly suppressed compared with that of the control group 8 [NIR(−) Pan‐IR700(−) device(−)] after 2 wk (P < .01) (Figure 5A). Tumor growth in groups 2 [NIR(−) Pan‐IR700(+) device(+)], 3 [NIR(−) Pan‐IR700(+) device(−)], 4 [NIR(+) Pan‐IR700(−) device(+)], 5 [NIR(−) Pan‐IR700(−) device(+)] and 7 [NIR(+) Tra‐IR700(+) device(+)] were similar to that of control group 8.
FIGURE 5.
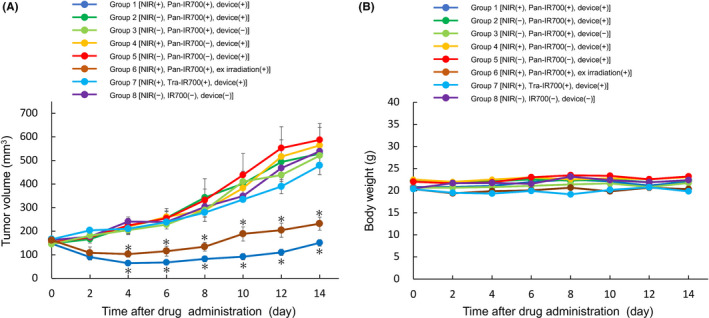
In vivo NIR‐PIT in mice: changes in tumor volume and body weight. A, Tumor growth curve in the 6 groups. The tumor volumes of group 1 [NIR(+) Pan‐IR700(+) Device(+)] and group 6 mice [NIR(+) Pan‐IR700(+) external irradiation(+)] were significantly suppressed compared with that in group 8 [NIR(−) IR700(−) Device(−)]. The other 4 groups did not show any therapeutic effects. Data are presented as the means ± SEM (n = 4, *P < .01 vs untreated control, Dunnett t test). B, There were no differences in body weight change between any groups during the experiment (n = 3, no significant difference vs untreated control, Dunnett's test)
We measured the body weight of mice in the in vivo experiments. There was no difference in body weight between groups until day 14 (Figure 5B). Although the site of catheter insertion left a small scar on all mice, the scar disappeared within several days. After NIR treatment with this device, 2 mice presented with a mild burn injury of the skin that was not considered significant, and this disappeared within 1 wk.
These results suggested that the novel wired NIR‐LED catheter was feasible for use as a light source for NIR‐PIT and was less invasive than current methods and was without systematic side effects.
3.5. Histological analysis
H&E staining of tumors in group 1 [NIR(+) Pan‐IR700(+) device(+)] demonstrated a significant therapeutic outcome, accompanied by microhemorrhage and cancer cell death. However, no obvious damage was observed in control group 8 mice [NIR(−) Pan‐IR700(−) device(−)]. (Figure 6). Taken together, the histological analyses suggested that NIR‐PIT using the developed NIR‐LED catheter induced therapeutic effects against CCA tumors.
FIGURE 6.
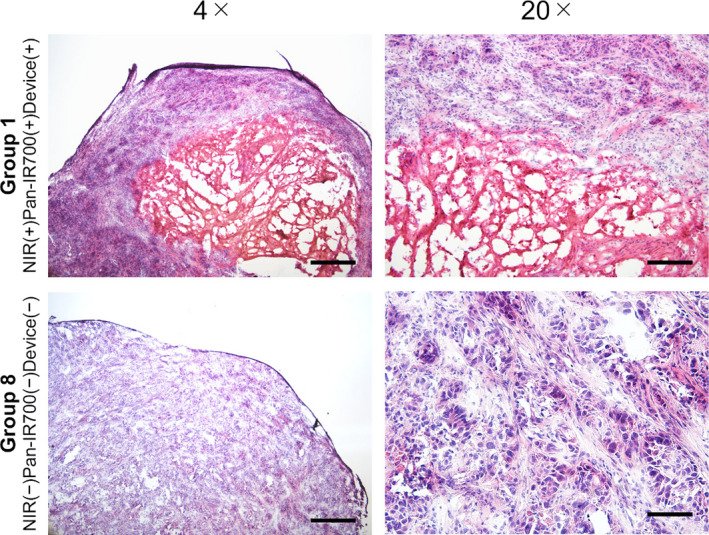
Histological analysis of NIR‐PIT treated tumors. In the tumors of group 1 [NIR(+) Pan‐IR700(+) Device(+)] mice, hemorrhage and the disappearance of tumor cells were observed at the center of the tumor. In contrast, no apparent damage was observed in group 8 [NIR(−) Pan‐IR700(−) Device(−)] mouse tumors. Scale bar: 500 µm in 4× images. Scale bar: 100 µm in 20× images
4. DISCUSSION
We revealed that NIR‐PIT for CCA was effective in EGFR‐ or HER2‐expressing CCA cells and that a novel catheter with LEDs was useful for NIR‐PIT of CCA.
In this study, the novel catheter continuously irradiated tumors with NIR light with minimal invasion and treated CCA tumors in mice. NIR‐PIT using the novel catheter was also shown to be as effective as conventional laser irradiation. This catheter was designed to be inserted into the bile duct under endoscopic retrograde cholangiography or by the percutaneous transhepatic route. By inserting this catheter to the bile duct, NIR‐PIT for extrahepatic or intrahepatic bile duct cancer and pancreatic head cancer would be feasible in clinical practice. Furthermore, by using our new device, similar to an endoscopic nasal bile duct drainage tube, the tumor can be continuously irradiated during the required treatment period. This would allow the therapeutic effects of NIR‐PIT to be enhanced depending on the light dose, as shown in the current and previous studies. 19 , 20 In addition, the super‐enhanced permeability and retention (SUPR) effect that induces enhanced vascular permeability and increased nano‐drug delivery by NIR‐PIT, would be expected for CCA, as shown in our in vivo experiment that indicated the re‐accumulation of Pan‐IR700 conjugates in IR700 fluorescence imaging. 21 Therefore, our novel catheter is capable of continuous irradiation and has advantages over current NIR‐PIT methods.
Many patients with CCA or pancreatic head cancer frequently suffer from obstructive jaundice and need endoscopic biliary drainage. 22 When this device is used for NIR‐PIT in patients with CCA, another drainage tube or stent will be needed for biliary decompression, because the developed new catheter lacked a drainage lumen. In the future, the device will be mounted with smaller LED tips and a biliary drainage lumen so that NIR‐PIT and biliary drainage can be performed concurrently. Furthermore, because low‐temperature burn was observed in our in vivo study, the LED catheter device should use a mounted temperature monitoring sensor to avoid thermal burn during NIR‐PIT. 23 Rather than using wired LED catheters, it would be desirable to develop a light‐irradiation stent‐like device that can be placed in conjunction with a tumor‐induced stenosis. This stent device would be less burdensome on patients than catheters, because the tube will not exit from the body. However, unlike a wired catheter, the method of power supply to irradiate light should be considered. In this context, we have previously developed an implantable wireless LED device for NIR‐PIT, 24 and this system might be a solution when developing the stent devices. We are currently developing similar devices that can be used more effectively and safely for clinical purposes.
In addition to the light source, targets on the membrane of cancer cells and specific antibodies are necessary for effective NIR‐PIT. NIR‐PITs targeting many types of cancers such as bladder, lung, and breast cancer have been reported in basic studies. 25 , 26 , 27 We believe that NIR‐PIT will also be an outstanding therapeutic option for CCA, as a previous study reported that about 40% of CCA have targetable genetic alterations that might be potential therapeutic targets. 28 In the present study, we demonstrated that EGFR‐ or HER2‐targeted NIR‐PIT was effective on CCA cell lines and we observed a relationship between target expression level and percentage of cell death. In addition of EGFR and HER2, Nishimura et al 29 showed the application of NIR‐PIT for CCA targeting tumor‐associated calcium signal transducer 2. Because NIR‐PIT is a therapy that does not rely on signal transduction and physically destroys target cells bound by IR700 conjugates, 30 the combination of our NIR‐LED device and appropriate antibody‐IR700 conjugates will enable NIR‐PIT on CCA cells.
This study had some limitations that should be resolved in the future. First, we used a xenograft murine model with a human CCA cell line. It was easy to evaluate the size of subcutaneously injected tumors and detect the IR700 fluorescence in them. In this study, we did not insert the catheter into the murine bile duct when performing NIR‐PIT, because it was too small. Detection of IR700 and the disappearance of its fluorescence after NIR‐PIT are important issues to address to confirm its therapeutic effect 18 , however we could not confirm this fact in the actual murine bile duct. Furthermore, we did not evaluate the operability of this catheter using endoscopy or a guidewire. Insertion of this catheter into the bile duct using a guidewire will allow us to evaluate the stability and durability of the catheter. Second, the immunogenic mechanism of the NIR‐PIT effect cannot be investigated in our xenograft murine model using human CCA cells. NIR‐PIT directly damages local cancer cells, but also activates host immune cells and reduces metastatic tumors. 11 Even if light from this catheter does not irradiate the whole CCA tumor, it can be radically targeted by the immune system. Therefore, an allograft murine model is needed to research the therapeutic effect of this treatment with regard to immunogenic cell death.
In conclusion, our findings demonstrated the beneficial effect of NIR‐PIT on CCA cells and the capability of the novel catheter device to perform NIR‐PIT. To obtain better outcomes using NIR‐PIT, appropriate antibody selection, method of biliary drainage during NIR‐PIT, and the safety and durability of the device should be considered. Moreover, the development of our novel catheter device will provide therapeutic benefit for the NIR‐PIT of CCA patients.
DISCLOSURE
HH and KM received funding from Japan Lifeline Co., Ltd. for this study. The funder was not involved in the study design, analysis, and interpretation of the data. OM received research funding from Rakuten Medical Japan, KK. The funder was not involved in the study design, analysis, and interpretation of the data.
AUTHOR CONTRIBUTIONS
HH mainly conducted experiments, performed the analysis, and wrote the manuscript. KN supported to conduct experiments, performed the analysis, and wrote the manuscript. YK and YY developed the catheter LED system. MK, MO, and NS planned and initiated the project, designed experiments, wrote the manuscript, and supervised the project.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This work was partly supported by the Japanese Foundation for Research and Promotion of Endoscopy (JFE) Grant, JSPS KAKENHI Grant Numbers 19H03593, the Uehara Memorial Foundation, JSPS Grant‐in‐Aid for JSPS Fellows Grant Numbers 20J12988, the Nagai Memorial Research Scholarship from the Pharmaceutical Society of Japan, and the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED under Grant Number JP19am0101093, the Platform Project for Supporting in Drug Discovery and Life Science Research (Platform for Drug Discovery, Informatics, and Structural Life Science) from the Japan Agency for Medical Research and Development (AMED), and Hokkaido University, Global Facility Center (GFC), Pharma Science Open Unit, funded by MEXT under “Support Program for Implementation of New Equipment Sharing System.” We also appreciate the contribution of Dr. Hirohisa Yano (Kurume University School of Medicine) for kindly providing the cell line, KMCH‐1.
Hirata H, Kuwatani M, Nakajima K, et al. Near‐infrared photoimmunotherapy (NIR‐PIT) on cholangiocarcinoma using a novel catheter device with light emitting diodes. Cancer Sci. 2021;112:828–838. 10.1111/cas.14780
REFERENCES
- 1. Waseem D, Tushar P. Intrahepatic, perihilar and distal cholangiocarcinoma: management and outcomes. Ann Hepatol. 2017;16:133‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nagakawa T, Kayahara M, Ikeda S, et al. Biliary tract cancer treatment: results from the Biliary Tract Cancer Statistics Registry in Japan. J Hepatobiliary Pancreat Surg. 2002;9:569‐575. [DOI] [PubMed] [Google Scholar]
- 3. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273‐1281. [DOI] [PubMed] [Google Scholar]
- 4. Pellat A, Vaquero J, Fouassier L. Role of ErbB/HER family of receptor tyrosine kinases in cholangiocyte biology. Hepatology. 2018;67:762‐773. [DOI] [PubMed] [Google Scholar]
- 5. Jain A, Javle M. Molecular profiling of biliary tract cancer: a target rich disease. J Gastrointest Oncol. 2016;7:797‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chong DQ, Zhu AX. The landscape of targeted therapies for cholangiocarcinoma: current status and emerging targets. Oncotarget. 2016;7:46750‐46767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee J, Park SH, Chang HM, et al. Gemcitabine and Oxaliplatin with or without Erlotinib in advanced biliary tract cancer: a multicentre, open label, randomised, phase3 study. Lancet Oncol. 2012;13:181‐188. [DOI] [PubMed] [Google Scholar]
- 8. Ramanathan RK, Belani CP, Singh DA, et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother Pharmacol. 2009;64:777‐783. [DOI] [PubMed] [Google Scholar]
- 9. Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011;17:1685‐1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakajima K, Takakura H, Shimizu Y, Ogawa M. Changes in plasma membrane damage including cell death after treatment with near‐infrared photoimmunotherapy. Cancer Sci. 2018;109:2889‐2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ogawa M, Tomita Y, Nakamura Y, et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget. 2017;8:10425‐10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kobayashi H, Choyke PL. Near‐infrared photoimmunotherapy of cancer. Acc Chem Res. 2019;52:2332‐2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kobayashi H, Griffiths GL, Choyke PL. Near infrared photoimmunotherapy: photo‐activatable antibody‐drug conjugates (ADCs). Bioconjug Chem. 2020;31:28‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim MM, Darafsheh A. Light sources and dosimetry techniques for photo dynamic therapy. Photochem Photobiol. 2020;96:280‐294. [DOI] [PubMed] [Google Scholar]
- 15. Nagaya T, Okuyama S, Ogata F, Maruoka Y, Choyke PL, Kobayashi H. Endoscopic near infrared photoimmunotherapy using a fiber optic diffuser for peritoneal dissemination of gastric cancer. Cancer Sci. 2018;109:1902‐1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee JY, Diaz RR, Cho KS, et al. Efficacy and safety of photodynamic therapy for recurrent, high grade nonmuscle invasive bladder cancer refractory or in tolerant to bacille Calmette Guerin immunotherapy. J Urol. 2013;190:1192‐1199. [DOI] [PubMed] [Google Scholar]
- 17. Euhus DM, Hudd C, LaRegina MC, Johnson FE. Tumor measurement in the nude mouse. J Surg Oncol. 1996;31:229‐234. [DOI] [PubMed] [Google Scholar]
- 18. Sano K, Nakajima T, Choyke PL, Kobayashi H. Markedly enhanced permeability and retention effects induced by photo immunotherapy of tumors. ACS Nano. 2013;22:717‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitsunaga M, Nakajima T, Sano K, Choyke PL, Kobayashi H. Near‐infrared theranostic photoimmunotherapy (PIT): repeated exposure of light enhances the effect of immunoconjugate. Bioconjug Chem. 2012;23:604‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakajima T, Sato K, Hanaoka H, et al. The effects of conjugate and light dose on photo‐immunotherapy induced cytotoxicity. BMC Cancer. 2014;14:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kobayashi H, Coyke PL. Super enhanced permeability and retention (SUPR) effects in tumors following near infrared photoimmunotherapy. Nanoscale. 2016;8:12504‐12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lai EC, Mok FP, Tan ES, et al. Endoscopic biliary drainage for severe acute cholangitis. N Engl J Med. 1992;326:1582‐1586. [DOI] [PubMed] [Google Scholar]
- 23. Okuyana S, Nagaya T, Ogata F, et al. Avoiding thermal injury during near‐infrared photoimmunotherapy (NIR‐PIT): the importance of NIR light power density. Oncotarget. 2017;8:113194‐113201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakajima K, Kimura T, Takakura H, et al. Implantable wireless powered light emitting diode (LED) for near‐infrared photoimmunotherapy: device development and experimental assessment in vitro and in vivo. Oncotarget. 2018;9:20048‐20057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nagaya T, Okuyana S, Ogata F, et al. Near infrared photoimmunotherapy targeting bladder cancer with a canine anti‐epidermal growth factor receptor (EGFR) antibody. Oncotarget. 2018;9:19026‐19038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sato K, Nagaya T, Mitsunaga M, Choyke PL, Kobayashi H. Near infrared photoimmunotherapy for lung metastases. Cancer Lett. 2015;365:112‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jin J, Krishnamachary B, Mironchik Y, Kobayashi H, Bhujwalla ZM. Phototheranostics of CD44‐positive cell populations in triple negative breast cancer. Sci Rep. 2016;6:27871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003‐1010. [DOI] [PubMed] [Google Scholar]
- 29. Nishimura T, Mitsunaga M, Sawada R, et al. Photoimmunotherapy targeting biliary‐pancreatic cancer with humanized anti‐TROP2 antibody. Cancer Med. 2019;8:7781‐7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sato K, Ando K, Okuyama S, et al. Photoinduced ligand release from a silicon phthalocyanine dye conjugated with monoclonal antibodies: a mechanism of cancer cell cytotoxicity after near‐infrared photoimmunotherapy. ACS Cent Sci. 2018;4:1559‐1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


