Figure 2.
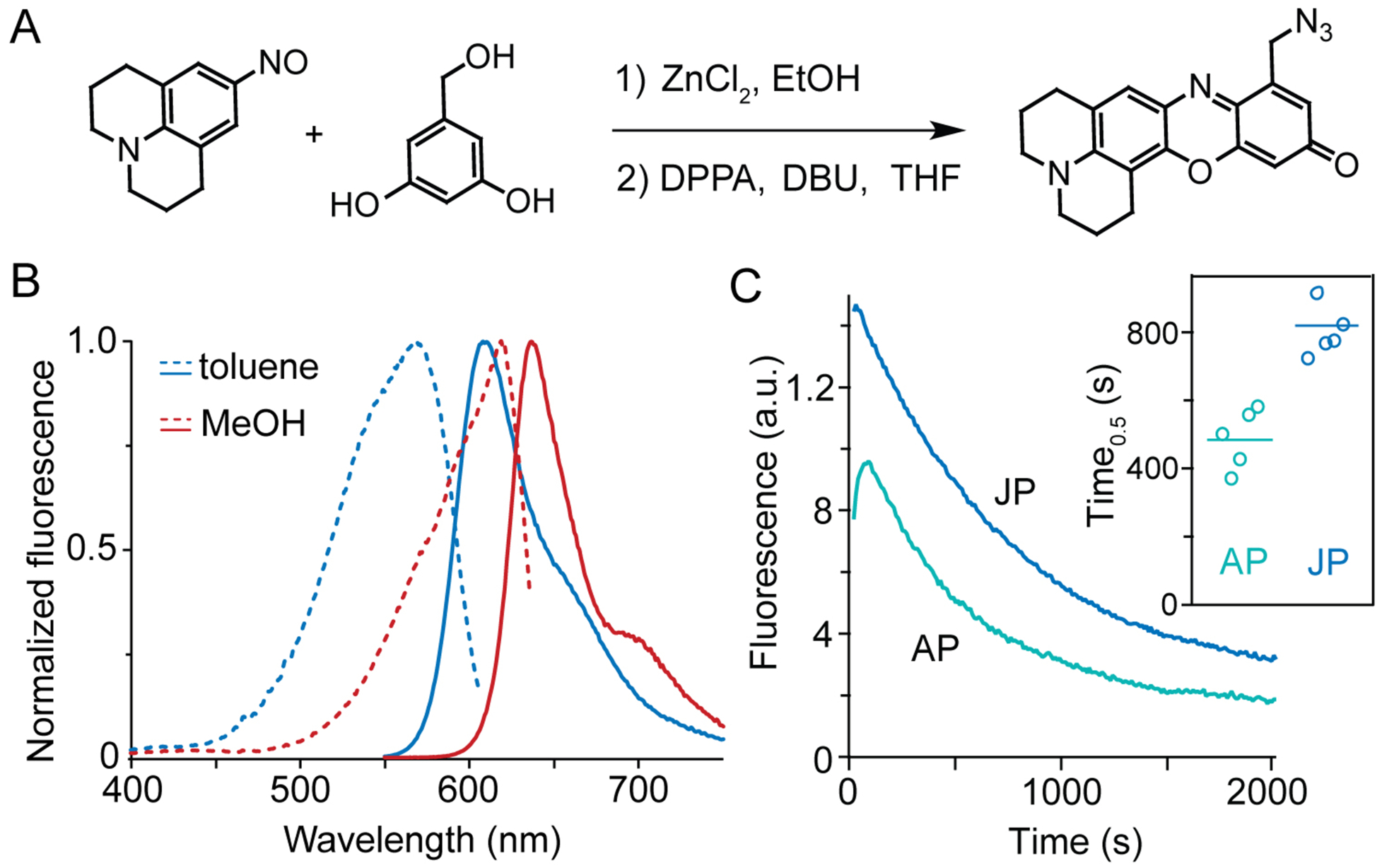
Synthesis and fluorescence of a far-red environment sensor. (A) Synthesis of JP-N3 by condensation of nitroso julolidine and hydroxymethyl resorcinol. DPPA: Diphenyl phosphorazidate; DBU: 1,8-diazabicyclo[5.4.0]undec-7-ene. (B) JP environment sensitivity. Excitation (dashed) and emission (solid lines) spectra of JP-N3 in nonpolar and polar solvents. (C) Enhanced brightness and stability (inset) conferred by julolidine rings. Emission measured by epifluorescence microscopy with 550-nm excitation of AP (ref31) or JP adducts to Lys27Pra on Kv2.1-expressing CHO cells.
