Figure 2.
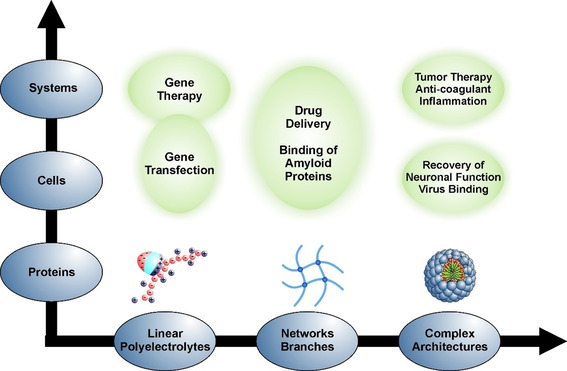
Interaction of polyelectrolytes with biosystems at different levels of complexity: Linear polyelectrolytes may be assembled into networks[ 55 , 56 ] and branched systems. Ultimately, they may become building blocks for systems with higher complexity, for example, micelles with core–shell structures. Complexity on the biological side starts with single protein molecules that can interact with polyelectrolyte systems with various architectures. On this level, the therapeutic activity of polyelectrolytes can often be traced back to a blocking of proteins by a suitable polyelectrolyte system.[ 31 , 36 , 39 , 40 , 42 , 45 ] Cells present the next level of complexity and their interaction with charged polymeric systems must be understood when considering these systems for, for example, drug delivery or gene transfection.[ 20 , 22 , 57 , 58 , 59 ] Organs present the highest level of complexity and the understanding of their interaction with synthetic polyelectrolyte systems is in its infancy. However, cationic polyelectrolytes with suitable architectures have recently been introduced as agents with anticoagulant reversal activity in blood.[ 41 , 42 , 43 ] The entire matrix of systems and problems gives a good overview of the possible medical problems to which synthetic polyelectrolytes may provide solutions.
