Abstract
Introduction
The essence of cardiac resynchronization therapy (CRT) is biventricular (BiV) pacing, which involves implanting pacing leads in both the right ventricle (RV) and left ventricle (LV). Unlike traditional RV pacing, many hurdles lie ahead of successful LV lead implantation.
Methods and Results
In this review, we first highlight the importance of optimizing the patient and the tools. Next, we describe the CRT tools developed over several decades, to facilitate successful implantation. Thereafter, we provide a streamlined step‐by‐step summary of the basic BiV implantation procedure. Lastly, we discuss some commonly encountered challenges during implantation and the techniques to tackle them.
Conclusion
A systematic approach to every step of the implantation process can reduce procedure time, decrease patient exposure to radiation and contrast, and minimize complications. The use of right tools and techniques can enable all implanters to become more successful with BiV implantation.
Keywords: Amplatz wire, cardiac resynchronization therapy, coronary sinus, CS cannulation, heart failure, left ventricular lead implantation, vein selector
1. INTRODUCTION
Cardiac resynchronization therapy (CRT) is one of the most important therapeutic advancements in recent years for patients with heart failure with reduced ejection fraction (HFrEF). Throughout the past two decades, numerous trials and studies have repeatedly illustrated the efficacy of CRT to improve outcomes in carefully selected patients. 1 However, not all HFrEF patients respond to CRT, with response rate quoted at 60%–70%. 2 Multiple factors have been shown to play roles in CRT response, including a native left bundle branch block (LBBB) morphology, non‐ischemic cardiomyopathy (NICM), sinus rhythm, a wider QRS duration, female gender, etc. 3 From the authors' perspective, one of the most important factors that determines CRT response is the ability to place the left ventricular (LV) lead at the desired location. This is supported by studies that examined the possibility of enhancing CRT response by optimizing LV lead location. 4 , 5 , 6 With the conventional approach it requires years of experience to become comfortable with LV lead implantation. However, in some cases, the target branch can still be difficult to reach. We believe that the processes, tools, and techniques described here can provide an alternative and innovative approach to successful biventricular (BiV) implantation. This article aims to provide a standardized and optimized approach to LV lead placement to improve success and minimize complications.
2. PROCESS OPTIMIZATION
Process optimization is essential to successful LV lead placement. This section discusses ways to optimize each step before LV lead placement.
2.1. The “BiV Cart”
It is imperative that operators are familiar with all available tools at their disposal. Collecting all implantation equipment before the procedure will enable a smooth process. The equipment specifically designed for LV lead placement is discussed below. We recommend having a full set of equipment sufficiently stocked in a “BiV Cart,” giving operators the freedom to concentrate on the procedure. An example of a “BiV Cart” used at the authors' institution is depicted in Figure 1A.
Figure 1.
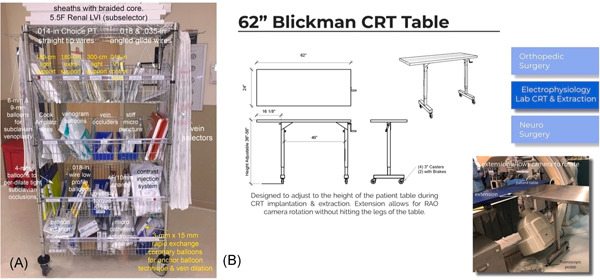
The “BiV Cart” and height adjustable table. (A) The biventricular (BiV) cart and (B) height adjustable table with extension
2.2. The table
2.2.1. Table height and drop‐leaf design
We recommend having an instrument table that is height adjustable and raised to the same level as the patient. From our experiences, we found that a 14–16‐in portion of the table can be extended beyond the legs on one side (either fixed or drop‐leaf). This extension allows room for the fluoroscopy camera to rotate without hitting the legs of the table (Figure 1B).
2.2.2. Instrument table orientation
Equally important to table height is table orientation. Having the instrument table in the comfortable orientation during various stages of the procedure is critical but occasionally neglected. In traditional lead implantation, the instrument table is behind the operators. However, with LV lead implantation the long wires and catheters required for the coronary venous anatomy lie on the patient's body and are particularly prone to falling off the table, increasing the risk of contamination. In addition, the complexity demands the operators to make more manipulations with the catheters and wires, increasing the risk of them falling off the table. As a result, we have reinforced the concept of placing the instrument table perpendicular to the patient during LV lead implantation. This position is preferred as soon as the operators begin to obtain venous access for the coronary sinus (CS). This way the long wires, sheaths, and guides can be kept straight as they exit the body and fall naturally on the instrument table.
Occasionally, we have to perform implantation from the right side of the patient. In these cases, we recommend having the table perpendicular to the patient and performing the procedure facing the patient's feet. This prevents a right‐handed operator from temporarily becoming “left‐handed” (Figure 2).
Figure 2.
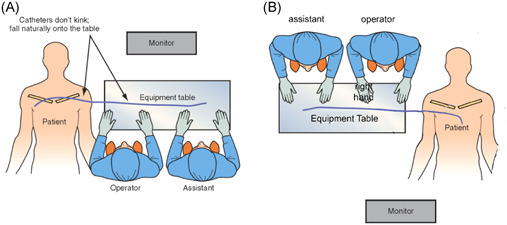
Table position during implantation. (A) The table is perpendicular to the patient during LV lead placement. This orientation improves ergonomics and prevents catheters from kinking and falling from the table. (B) During a right‐sided implantation, the operators should face the patient's feet to allow better control with the dominant hand (right hand in this case). LV, left ventricle
2.3. The patient
2.3.1. Preprocedural hydration to prevent contrast‐induced nephropathy (CIN) and increase central venous pressure (CVP)
CIN is a common complication of any procedure that involves the use of intravenous contrast agents, including pacemaker implantation. In our experience, the best approach to prevent CIN is adequate preprocedural hydration rather than limiting the injection of contrast agents. Limiting contrast injection can often lead to underestimation of the options provided by coronary venous anatomy, thereby preventing speedy execution of lead placement and requiring more overall contrast exposure.
Since the volume status of patients indicated for CRT is variable, we recommend hydration with normal saline for all patients. Although concerns for volume overload can be a deterrence to this approach, we believe volume overload secondary to our hydration protocol is rare and is offset by the advantage of preventing CIN. It is important to note that this approach is based on experience from our center and the volume status of each patient still needs to be taken into consideration before the initiation of hydration. For patients with underlying heart failure, additional saline should be used with caution due to limited evidence. Below is the standard hydration protocol we recommend to our patients undergoing CRT implantation:
Normal saline 3 ml/kg/h starting 1 h before the procedure (typically as the patient leaves the holding area) and continued at 1 ml/kg/h during the procedure and continuing 6 h after the procedure.
To prevent volume overload, it is important that hydration not be started until the patient is being transported from the holding area to the procedure room (not when the patient leaves the floor for the electrophysiology [EP] Lab). This way, the 1‐h saline bolus is completed just as the procedure starts.
2.3.2. Elevation of patient's legs or Trendelenburg position to optimize venous access and to prevent pneumothorax
A successful initial venous access can sometimes make all the difference in lead placement. Elevating the patient's legs with a large triangular cushion (wedge) or having the patient in the Trendelenburg position elevates the patient's CVP and makes the central vein an easier target for the venous stick. This technique also minimizes the risk of pneumothorax. Once venous access is completed, the cushion is removed.
3. INTERVENTIONAL TOOLS FOR LV LEAD IMPLANTATION
Over the years, LV pacing leads and delivery systems have improved dramatically. However, these advances are often not adequate to accommodate variations in patients' coronary venous anatomy. The authors have developed an approach based on interventional principals by adapting existing equipment to device implantation. However, complete implementation required the development of a new four‐component lead delivery system. This section describes each component of the CS Catheter Delivery System (Figure 3) in detail.
Figure 3.
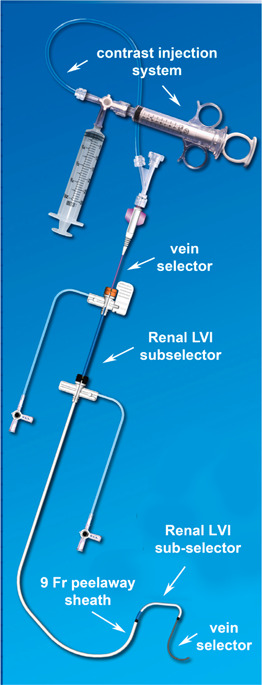
The CS catheter delivery system. The delivery system is comprised of a contrast injection system connected to the vein selector, a renal LVI sub‐selector, and a 9‐F peel‐away sheath. CS, coronary sinus; LVI, lateral vein introducer
3.1. CS access catheter and CS telescoping cannulation assist catheter
The CS access catheter provides a stable 9‐F inner diameter (ID) peel‐away platform of support for CS cannulation. This 9‐F ID is particularly important when patients have difficult coronary venous anatomy. The telescoping CS cannulation assist catheter has a braided core to provide torque control and stability, which allows direction and advancement of the CS peel‐away access catheter.
3.2. Vein selector
The vein selector is a braided 5‐F outer diameter (OD), 75 cm long catheter with a soft tapered tip designed to locate the target vein with contrast injection, deliver a guidewire into the vein, and serve as a rail over which the delivery guide (sub‐selector) can be advanced into the target vein. With experience, the vein selector shapes have been distilled down to the Standard, the Hook, and the Vert (Figure 4A). More than 60% of cases can be performed with the Standard Vein Selector. The CS Hook is useful for branches that take off in close proximity to the CS ostium and ones with an extremely acute takeoff. The CS Vert is preferred when the takeoff is less acute but more tortuous. These vein selectors are telescoped inside the delivery guides (sub‐selectors) of most manufacturers.
Figure 4.
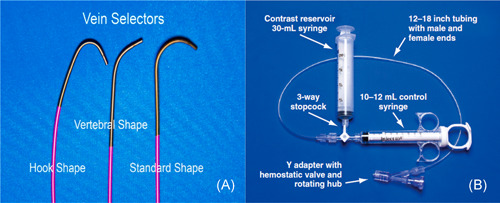
Vein selectors and contrast injection system. (A) Vein selectors. Different shapes from left to right: Standard, Vert, and Hook. (B) The contrast delivery system. For a right‐handed operator, the right hand is on the rotating hub on the Y adapter, and the left hand is on the catheter
3.3. Lateral vein introducer
The lateral vein introducer (commonly known as a sub‐selector) is a braided catheter designed to provide support for inserting the LV lead directly into the target vein (not for CS access). Sub‐selectors are specifically designed for lead delivery thus, it can be difficult to use them to engage and/or advance into a target branch even using a wire. Having the vein selector telescoped inside the sub‐selector enhances the sub‐selector. Most device companies provide a sub‐selector for LV lead delivery.
3.4. Contrast injection system
The contrast injection system consists of a 30 ml contrast reservoir syringe, a 10–12 ml control syringe, a 3‐way stopcock, a 12–18 in tubing with male and female ends, and a Y adapter with hemostatic valve and rotating hub (Figure 4B). When occlusive venogram is needed, we prefer a balloon with 0.035 in lumen instead of 0.018 in. Here, we recommend using a syringe that has a reinforced plunger, stiffer side wall, and smaller inner diameter compared to conventional 10 ml syringes. This design enables the injection of sufficient contrast against vessel resistance at the necessary flow rate (120 ml/min vs. 46 ml/min with a normal 10 ml syringe).
4. BASIC BIV IMPLANTATION PROCEDURE USING THE CS CATHETER DELIVERY SYSTEM: A STEP‐BY‐STEP SUMMARY
After process optimization, the procedure begins. We believe a systematic approach to every step of the procedure can minimize errors, reduce procedure time, and decrease patient exposure to radiation and contrast. Over the years, we have developed our own approach for LV lead placement. This section describes a step‐by‐step protocol of the basics of LV lead implantation, using the CS Catheter Delivery System:
-
Prepare the implant table
Assemble contrast injection system
50 ml of undiluted contrast
Elevate the patient's legs to increase CVP
-
Obtain venous access using contrast
Prep and drape the implant site
Position the implanting physician, X‐ray, and the needle
Inject 10–20 ml of full‐strength contrast and flush with 30–50 ml of normal saline
Stick the target vein with the contrast flowing
-
Obtain three separate axillary vein access
Two short wires
One long wire
Coil the long wire and clip it to the drapes
Insert the right ventricular (RV) and right atrial (RA) leads
Turn the implant table perpendicular to the patient and raise the table to patient
Assemble the contrast injection system (the operator will use BOTH hands for catheter manipulation and the assistant will inject contrast)
Position the screen to a comfortable position
Unclip the long wire and insert the long peel‐away sheath/dilator
Fix the dilator when the tip reaches the mid‐sternum
Advance the sheath off the dilator and withdraw the wire until the tip of the sheath enters the RV
Attach the braided core to the injection system, clear with contrast and insert into the sheath until the black mark on the braided core reaches the hub of the sheath. At this point the tip of the braided core will be at the tip of the sheath. Withdraw the sheath over the braided core until 1 cm of braided core remains uncovered
Apply gentle counterclockwise torque to the rotating hemostatic valve and braided core to direct the tip posterior (which will generate premature ventricular contractions [PVCs] if the tip is in the RV)
Maintaining gentle counterclockwise torque withdraw the sheath and braided core as a unit until the tip of the braided core reaches the tricuspid annulus (PVCs no longer present)
Stop withdrawing
Drop the tip of the braided core down the tricuspid annulus by application of additional counterclockwise
When the tip stops dropping, perform a 1–2 ml test puff of full‐strength contract
Locate the CS and cannulate the CS with the braided core and sheath (add a wire if needed)
Perform an occlusive CS venogram
Select the vein and decide on lead shape and size
Choose the appropriate SHAPE of the vein selector
Use the “Renal” shape lateral vein introducer (LVI) sub‐selector 5.5‐F ID for leads ≤ 5 Fr
Load the vein selector into the Renal LVI sub‐selector
Attach the injection system to the vein selector
Insert the Renal LVI/vein selector combination into the CS access catheter
Advance the Renal LVI until it reaches the tip of the CS access catheter
Advance the vein selector out of the Renal LVI into the CS
It is important that the injection system with rotating hemostatic valve is attached to the vein selector (NOT JUST A Syringe)
Use both hands for control and watch the tip of the vein selector. With practice, the vein selector can be moved safely without a wire within the CS and its branches
Rotate the vein selector laterally just above the target (based on CS venogram)
-
Have an assistant gently inject 1 ml of contrast if either of the events below occurs
The tip drops out of the curve of the CS; or
The tip stops moving freely
Insert a floppy 0.014 in polymer tip hydrophilic angioplasty wire into the vein as far as it will go
Inject 1 ml contrast to confirm the wire is in the main body of the target vein
Advance the vein selector over the wire slightly into the vein
Insert an extra‐support 0.014 in polymer hydrophilic angioplasty wire into the vein
After confirming the position of the wires, advance the vein selector further over the two wires to a stable position deep in the vein
Hold the wires and vein selector stable, and advance the Renal LVI
Rotate Renal LVI sub‐selector gently as it approaches the ostium of the vein
Advance/withdraw the wire stabilized vein selector as needed to advance the Renal LVI sub‐selector deep into the vein
If needed, a third and fourth wire can be inserted into the vein selector for stability
Make sure again the equipment table is perpendicular to and at the same height as the patient
Remove the vein selector initially and retain all the wires
The ID of the new 5.5‐F Renal LVI is too small to allow a buddy wire thus the extra wires must be removed retaining the extra support wire for lead delivery
Advance the lead into the vein over the wire, remove the wire and test
With the Renal LVI secure within the target vein, advance a soft stylet to the tip of the lead
Advancing the stylet to the tip of the lead often makes it possible to advance the lead further into the vein and improves stability while removing the delivery system (Video S1)
Position the tip of the sheath in the proximal to mid‐CS
-
Position the patient under anterior–posterior (AP) fluoroscopy, such that
The tip of the lead is at the right edge of the screen (not in the middle)
The CS ostium is in the middle of the screen
RA is visible on the left side of the screen
Slice the delivery guide
-
Remove the peel‐away sheath
First DO NOT crack the hub
While watching the lead under fluoroscopy, fix the lead and withdraw the sheath over the lead
Watching the RA add or remove slack as needed
-
When the hemostatic hub of the sheath reaches the IS‐1 connector
Have the assistant compress the walls of the sheath against the lead
Crack the hub and peel down to the assistant's fingers
Slide the remaining sheath back until it exits the lead
Have the assistant secure the lead
Finish peeling the sheath
Remove the stylet quickly (like starting a lawn mower) until the tip of the stylet is at mid‐sternum
Adjust lead slack in the LAO projection: the lead should follow the bottom of the RA then up the lateral wall of the RA to the SVC
Secure the lead using the “tie to the knot technique” not directly to the muscle. Twiddler syndrome is a physician problem not a patient problem (Video S2).
5. TECHNIQUES TO TACKLE COMMONLY ENCOUNTERED CHALLENGES DURING LV LEAD IMPLANTATION
One of the most important yet challenging aspects of CRT is achieving transvenous pacing of the LV epicardium. However, many obstacles lie ahead of successful implantation. These can include but are not limited to stenotic or occluded subclavian vein due to prior pacemaker implantations, difficulty locating the CS, tortuous CS, and difficulty finding an appropriate target vein to secure the LV lead, etc. Even when the LV lead is implanted in the target vein, other problems such as high‐pacing thresholds, phrenic nerve pacing, and lead dislodgement during retrieval of the delivery system can jeopardize success. This section briefly talks about ways to tackle various difficult cases.
5.1. Locating and cannulating the CS using the anatomic approach
One of the first hurdles to overcome for LV lead placement is CS cannulation. If approached from the RA, CS cannulation can be challenging when the Eustachian ridge and the Thebesian valve prevent the catheter from entering the CS. Interestingly, when the tip of the catheter approaches the CS via dropping down the tricuspid anulus from the RV by application of counterclockwise torque, the ridge and valve help direct the tip into the CS. As a result, it is imperative that the tip of the catheter is across the Eustachian ridge and above the Thebesian valve for successful cannulation.
5.2. Locating and cannulating the CS in patients with a dilated RA
When the curve of the catheter is too small for the RA anatomy, the tip will be below the CS ostium and on the atrial side of the Eustachian ridge. With counterclockwise torque, the catheter will move away from the CS. These circumstances occur when patients have massively dilated RA and the standard catheters do not provide enough length and curvature to position the tip across the Eustachian ridge and above the Thebesian valve. Catheters with bigger curvatures are recommended (e.g., the Jumbo catheter). Figure 5 shows the extended curvature of the jumbo catheter compared to the standard sheath. Therefore, in cases when patients have massively dilated RA, catheters with bigger curvatures should be used to aid with locating the CS.
Figure 5.
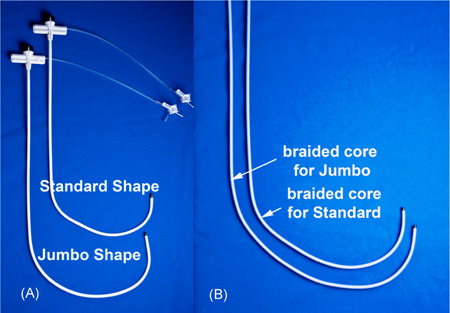
Standard delivery sheath and the Jumbo sheath. The Jumbo sheath has a much bigger curvature compared to the standard sheath to reach beyond the Thebesian valve and Eustachian ridge in patients with dilated RA to better identify the CS ostium. Standard delivery sheaths from other vendors can also be used. CS, coronary sinus
5.3. Difficult CS cannulation: Amplatz CS cannulation technique
Once the opening of the CS has been located and successfully accessed, the next step is to advance the sheath deeper into the CS in search for potential target veins. This seemingly straightforward step can be complicated by several anatomical variations: (1) the target vein takeoff is at the CS ostium, (2) the target vein has a difficult takeoff angle near the CS ostium, (3) the target vein drains into the middle cardiac vein, (4) CS access is unstable typically in patients with massive RA, and (5) tortuous or stenotic CS. Here we introduce the Amplatz Wire CS Cannulation technique to specifically deal with these issues.
The rationale behind this technique is to create a rail strong enough to support the advancement of the sheath. We utilize the J‐tip short taper Amplatz Extra Stiff (0.035 in, 180 cm, 3 mm J tip short taper from Cook, Merit Medical, or other vendors) because the body of the wire is stiff enough to provide support for the sheath. Moreover, the J‐tip short taper Amplatz wires transition from floppy to stiff right after the J‐tip (Figure 6). This “short taper” design provides more support to the sheath as the wire within the CS is stiff. The standard taper Amplatz wires transition from floppy to stiff approximately at the ostium of the CS. This setup tends to displace the floppy wire within the CS.
Figure 6.
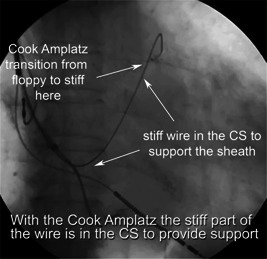
The Amplatz wire CS cannulation technique. To achieve CS cannulation, we utilized the vertebral vein selector and the Cook Amplatz wire to provide support to the sheath. Notice here, the Cook Amplatz wire transitions from floppy to stiff rapidly after the J tip, providing greater support compared to the traditional Amplatz wire that transitions at a much later point (white arrows). CS, coronary sinus
Attempting to advance a J‐Tip short taper Amplatz wire into the distal CS (great cardiac vein/proximal anterior interventricular vein [AIV]) tends to push the sheath out of the CS. As a result, we use a 0.035 in angled glide wire plus a vertebral vein selector to ensure the stiff wire safely reaches a stable position deep in the CS (great cardiac vein/AIV junction).
The following summarizes the steps of the Amplatz wire CS cannulation technique:
Open the hemostatic valve of the Y adapter attached to the braided core
While avoiding the vein of Marshall (VOM) advance the angled 0.035 in glide wire into the AIV
Advance the vertebral vein selector through the hemostatic valve over the glide wire until its tip is in the AIV or distal great cardiac vein
Retain the vein selector position and remove the glide wire
Advance the short taper J tip Amplatz wire through the vein selector to the distal CS
Holding the vein selector and Amplatz wire in position, advance the braided core/sheath over the rail created by the Amplatz stabilized vein selector
Once the sheath is at mid‐CS, remove the vein selector and braided core while retaining the Amplatz wire
Secure the proximal end of the Amplatz wire by clipping it to the drape or edge of the table.
5.4. Proximal target veins and general sheath stability: Amplatz support wire technique
In situations where it is easy to cannulate the CS but there is a target branch near the CS ostium or additional support from the sheath is needed to advance the lead into the target vein, the Amplatz support wire technique is a valuable option. The same general principles that apply for the Amplatz CS cannulation technique apply to the Amplatz support wire technique. The security provided by having an Amplatz wire deep in the CS stabilizing the sheath is so compelling that many implanters use it routinely. An easier way to build the support wire into your workflow is to use a 0.035 in lumen balloon for venogram. When the venogram is completed, advance the balloon into the AIV over an angled glide wire, remove the glide, place the short taper Amplatz in the AIV through the balloon, and then remove the balloon retaining the Amplatz wire in the AIV. Clip the Amplatz to the drape or height adjustable table.
5.5. Lack of target vein options
In the modern era of CRT, implanters are becoming more specific about where to place the LV lead. According to consensus, the mid‐lateral wall of the LV is often regarded as the best location for LV resynchronization. As a result, the operator needs to have a good understanding of the patient's coronary venous anatomy before selecting the target vein. A set of well‐performed coronary venograms, specifically an occlusive CS venogram with full‐strength contrast from at least two views, preferably three (anterior–posterior, left anterior oblique, and right anterior oblique), is essential to visualize the branches that can be potential candidates to advance the LV lead. One of the major causes of unsuccessful LV lead implantation is the failure to recognize viable target vein options, due to an inadequately performed CS venogram.
5.5.1. CS venogram technique
Once the CS is cannulated, the operator can advance and inflate a 0.035 in lumen angiographic balloon catheter into the CS and inject full‐strength contrast to visualize the coronary venous anatomy. Importantly, achieving adequate occlusion will not only save time but also prevent complications such as CIN. If the balloon does not occlude the proximal or mid‐CS, it can be advanced further into the CS (typically beyond the Vieussens valve) and then inflated. The vessels proximal to the occlusion can be visualized during retrograde filling of contrast and by releasing the balloon at the end of the injection (Videos S3 and S4).
5.5.2. Vein selector venograms to reveal side branches and alternative lateral wall branches
Under some circumstances, even an adequately performed two‐view, full‐strength occlusive coronary venogram might not reveal all the target veins for LV lead implantation. In this case, we recommend advancing the vein selector of the telescoping delivery system discussed above over a wire into the already identified branches and injecting full strength contrast through the vein selector to further characterize potential subbranch targets.
5.5.3. When is an occlusive venography dangerous?
CS venography is generally safe with a compliant occlusive balloon and when the tip of the balloon is observed during a test injection before initial balloon inflation. Contrary to conventional wisdom, it is not safe to inflate a balloon advanced over a wire before a test injection. When the balloon is advanced over a wire into a small branch or the VOM and inflated before a test injection, it will rupture the vein resulting in extravasation. When an occlusive venogram is performed, it may uncover prior unrecognized CS trauma typically caused by an EP catheter inadvertently advanced into the VOM or a lateral wall branch. During balloon inflation, the increased venous pressure distal to the balloon forces contrast into the damaged area, leading to aneurysm formation. If the aneurysm ruptures, blood and contrast escape into the pericardial space resulting in hemodynamic compromise, as illustrated in Figure 7.
Figure 7.
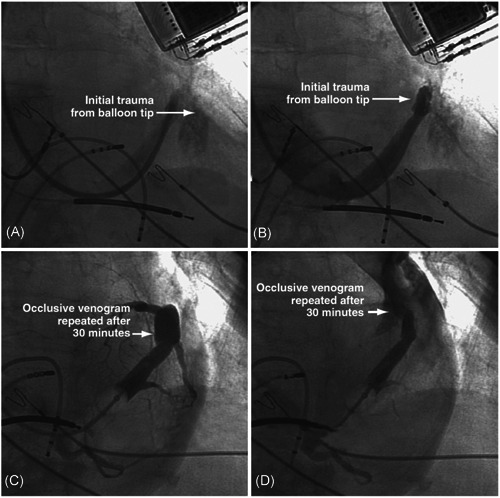
CS aneurysm formation and rupture with second occlusive venogram. (A, B) Trauma of the CS caused by the tip of the balloon. (C) An aneurysm of the CS was identified by occlusive venogram performed 30 min after the initial trauma. (D) The aneurysm ruptured with balloon occlusion and contrast injection, causing blood and contrast extravasation into the pericardial space
5.6. Anatomical variations
Another obstacle to successful LV lead placement is insufficient tools to deal with the anticipated variability of coronary venous anatomy. When precise lead implantation is required for optimal outcomes, navigating the anatomical variations without adequate tools is technically challenging and prone to complications. Structures like the Thebesian valve, the Vieussens valve, the VOM, and anatomic variations of the CS proper can breed the existence of coronary venous dissection and perforation. Here we discuss a few real‐life examples of how complications can occur in selected settings and approaches to avoid them.
5.6.1. The VOM and the Vieussens valve
The ligament of Marshall is described by Johan Marshall in 1850 as the remnant of the left common cardinal vein. 7 Continuous with the ligament is the VOM or oblique vein of the left atrium that drains into the proximal CS. The Vieussens valve is a membranous structure at the junction between the CS and the great cardiac vein. Not only is it an important landmark, the Vieussens valve also blocks catheter access to the great cardiac vein and tends to direct an incoming guidewire or EP catheter to the nearby VOM. When the operator mistakes the VOM for the main CS and tries to inflate a balloon or advance a sheath, dissection and perforation will occur. As pointed out above, the dissection may not become apparent until a venogram is performed. If the Vieussens valve is patent and the balloon is inflated proximally, the valve prevents contrast from entering into the great cardiac vein and forces contrast into the VOM or other small branches, causing venous rupture and extravasation. Therefore, it is preferable to perform occlusive venogram distal to the valve such that the anterograde flow of contrast illuminates distal branches while the retrograde filling of contrast reveals the proximal ones. Video S5 shows examples of dissection of the CS secondary to advancing the sheath into the patent VOM over an EP catheter.
5.6.2. The persistent left superior vena cava
Normally with development, the left superior vena cava (LSVC), which drains into the CS distal to Vieussens valve, involutes and becomes the ligament or VOM. However, in some patients the LSVC does not involute. The result is a persistent left superior vena cava (PLSVC). The increased venous flow from the left subclavian vein down the PLSVC into the CS distal to the Vieussens valve causes the proximal CS of these patients to be massively dilated. The dilated CS before Vieussens valve prevents adequate occlusive venogram, thereby increasing the difficulty of locating the target branches even with large injections of contrast. It is important to note that although the CS proximal to Vieussens valve is dilated, the CS above or distal to the Vieussens valve (technically the great cardiac vein) sees no increase in blood flow and remains normal in diameter. When the CS is entered from the RA, the CS distal to Vieussens valve (great cardiac vein) can be entered by directing the catheter laterally. In these cases, vein selectors can be tremendously helpful to direct the delivery system laterally to the PLSVC. Once inside the great cardiac vein, one way to effectively visualize target branches in these patients is to perform occlusive venogram beyond the valve. The anterograde flow of contrast will reveal distal target branches and the retrograde filling phase will show the proximal ones. The angled tips of the vein selectors can also easily engage branches above the Vieussens valve. Another way to visualize the branches is to probe the middle cardiac vein with the vein selector near the ostium of the CS using the Amplatz support wire technique. Once the middle cardiac vein is engaged, contrast injection through the vein selector will uncover potential target veins (Figure 8).
Figure 8.
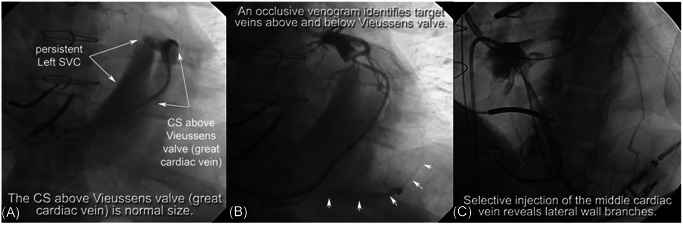
Cannulating the CS in patients with PLSVC. (A) In this case, the patient has a PLSVC and the CS is greatly dilated. However, the great cardiac vein as shown in fluoroscopy remains its normal size. (B) An occlusive venogram performed above the Vieussens valve identifies target veins both above and below the valve. (C) Selective injection of contrast in the middle cardiac vein is another approach to identify target branches. CS, coronary sinus; PLSVC, persistent left superior vena cava
6. CONCLUSION
CRT is one of the cornerstone therapies for patients with HFrEF. However, many obstacles lie ahead of successful and complication‐free LV lead implantation. Over the years, we have developed new tools and refined techniques to improve success. We are confident that using the processes, interventional tools, and techniques described here can help improve the success rate of BiV implantation. Additionally, we believe that many patients with previously failed attempts at LV lead implantation, using the interventional tools and techniques described here will be able to successfully undergo BiV implantation, and reap the benefits of CRT.
CONFLICT OF INTERESTS
Dr. Seth J. Worley receives royalties from Pressure Products and Merit Medical. The remaining authors declare that there are no conflict of interests.
Supporting information
Supporting information.Video 1. The Soft Stylet Can Be Used to Advance The LV Lead Deeper. https://youtu.be/SecHgqCnWJs.
Supporting information.Video 2. How to Tie Down The Suture Sleeve. https://www.youtube.com/watch?v=awHRDSygs4E.
Supporting information.Video 3. Failure to Perform Occlusive CS Venogram. https://www.youtube.com/watch?v=JCLl2m0VSJM.
Supporting information.Video 4. How to Perform An Adequate Occlusive CS Venogram. https://youtu.be/cfCiP2oVfUw.
Supporting information.Video 5. The Vieussens Valve and the VOM in CS Cannulation. https://www.youtube.com/watch?v=mQexUw5-VU4.
Zou F, Brar V, Worley SJ. Interventional device implantation, Part I: Basic techniques to avoid complications: A hands‐on approach. J Cardiovasc Electrophysiol. 2021;32:523‐532. 10.1111/jce.14748
Fengwei Zou and Vijaywant Brar contributed equally to this study.
Disclosures: None.
REFERENCES
- 1. Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845‐1853. 10.1056/NEJMoa013168 [DOI] [PubMed] [Google Scholar]
- 2. Birnie DH, Tang ASL. The problem of non‐response to cardiac resynchronization therapy. Curr Opin Cardiol. 2006;21(1):20‐26. 10.1097/01.hco.0000198983.93755.99 [DOI] [PubMed] [Google Scholar]
- 3. Rickard J, Michtalik H, Sharma R, et al. Predictors of response to cardiac resynchronization therapy: a systematic review. Int J Cardiol. 2016;225:345‐352. 10.1016/j.ijcard.2016.09.078 [DOI] [PubMed] [Google Scholar]
- 4. Khan FZ, Virdee MS, Palmer CR, et al. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J Am Coll Cardiol. 2012;59(17):1509‐1518. 10.1016/j.jacc.2011.12.030 [DOI] [PubMed] [Google Scholar]
- 5. Saba S, Marek J, Schwartzman D, et al. Echocardiography‐guided left ventricular lead placement for cardiac resynchronization therapy results of the speckle tracking assisted resynchronization therapy for electrode region trial. Circ Heart Fail. 2013;6(3):427‐434. 10.1161/CIRCHEARTFAILURE.112.000078 [DOI] [PubMed] [Google Scholar]
- 6. Daya HA, Alam MB, Adelstein E, et al. Echocardiography‐guided left ventricular lead placement for cardiac resynchronization therapy in ischemic vs nonischemic cardiomyopathy patients. Heart Rhythm. 2014;11(4):614‐619. 10.1016/j.hrthm.2014.01.023 [DOI] [PubMed] [Google Scholar]
- 7. Marshall J. On the development of the great anterior veins in man and mammalia; including an account of certain remnants of fætal structure found in the adult, a comparative view of these great veins the different mammalia, and an analysis of their occasional peculiarities in the human subject. Philos Trans R Soc London. 1850;140:133‐169. 10.1098/rstl.1850.0007 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.Video 1. The Soft Stylet Can Be Used to Advance The LV Lead Deeper. https://youtu.be/SecHgqCnWJs.
Supporting information.Video 2. How to Tie Down The Suture Sleeve. https://www.youtube.com/watch?v=awHRDSygs4E.
Supporting information.Video 3. Failure to Perform Occlusive CS Venogram. https://www.youtube.com/watch?v=JCLl2m0VSJM.
Supporting information.Video 4. How to Perform An Adequate Occlusive CS Venogram. https://youtu.be/cfCiP2oVfUw.
Supporting information.Video 5. The Vieussens Valve and the VOM in CS Cannulation. https://www.youtube.com/watch?v=mQexUw5-VU4.


