Fig. 1.
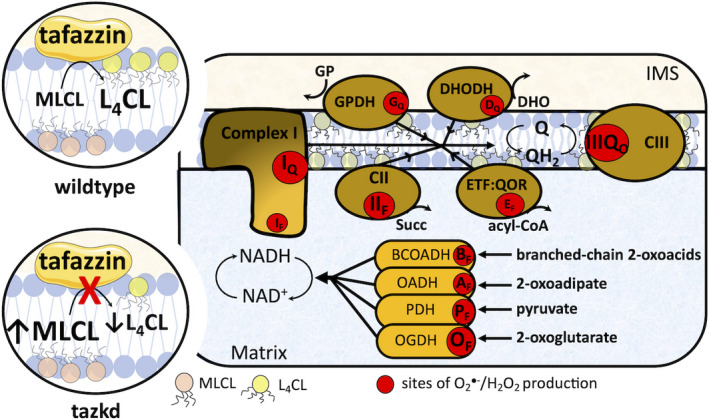
Tafazzin deficiency and the sites of superoxide/H2O2 in the mitochondria. Tafazzin is an acyltransferase important for remodeling CL to its physiologically relevant form, tetralinoleoyl CL (L4CL) (circle on top left, wild‐type). Mutations in the tafazzin gene alter the CL profile in the mitochondria and increase the levels of the intermediate, MLCL (circle on bottom left, tazkd). CL tightly interacts with and stabilize components of the electron transport chain (ETC), and aberrant CL profile is associated with higher mitochondrial superoxide () and hydrogen peroxide (H2O2) production. There are 11 sites with the capacity to produce /H2O2 in mitochondria. These sites are associated with the ETC and substrate oxidation enzymes and are represented by red circles. Reduced substrates from metabolism are transported into the mitochondria where they are oxidized; the electrons enter the ETC via enzymes that operate in close proximity to the NADH/NAD+redox potential and enzymes that operate in close proximity to the QH2/Q redox potential. The electrons flow from the NADH‐ and Q‐pool to complex III then to cytochromecand to complex IV, which finally transfers four electrons to oxygen, producing water. The sites associated with the enzymes in the NADH isopotential group and able to generate/H2O2 are the flavin/lipoate of the dehydrogenases of branched‐chain 2‐oxoacids (BCOADH, BF), 2‐oxoadipate (OADH, AF), pyruvate (PDH, PF), 2‐oxoglutarate (OGDH, OF), and the flavin site of complex I (IF). The sites in the Q isopotential group are the flavin site of complex II (IIF) and the electron transfer flavoprotein (ETF) and ETF:ubiquinone oxidoreductase (ETF:QOR) system (EF) and the ubiquinone binding sites of dehydrogenases of glycerol phosphate (GPDH, GF) and dihydroorotate (DHODH, DQ) and the outer quinol site of complex III, IIIQo. Electrons are transferred from the NADH‐ to the Q‐poolviasite IQin complex I, which has a high capacity for/H2O2 production. The diameters of the red circles are roughly proportional to their mean capacity for/H2O2 generation in heart and skeletal muscle. IMS, intermembrane space; CII, complex II; CIII, complex III.
