Scheme 1.
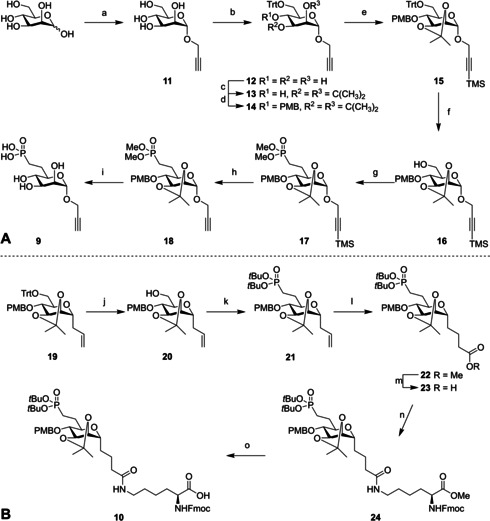
Synthesis of alkyne building blocks 9 and 10. a) i: Ac2O, pyridine; ii: propargyl alcohol, BF3 ⋅ OEt2, 50 °C; iii: NaOMe, MeOH, 70 % over three steps; b) TrtCl, Et3N, DMF, 60 °C, 83 %; c) p‐toluenesulfonic acid, 2,2‐dimethoxypropane, 87 %; d) p‐methoxybenzyl chloride, NaH, DMF, 95 %; e) TMSCl, nBuLi, THF, −78 °C, 97 %; f) i: p‐toluenesulfonic acid, CH2Cl2/MeOH; ii: p‐toluenesulfonic acid, 2,2‐dimethoxypropane; iii: 1 M HCl, EtOAc, 0 °C, 98 % over three steps: g) i: Tf2O, pyridine, CH2Cl2, −40 °C; ii: nBuLi, dimethyl methylphosphonate, THF, −70 to −50 °C, 72 % over two steps; h) TBAF, THF, quant.; i) i: TMSBr, pyridine, MeCN; ii: AcOH/H2O, 90 °C, 81 % over two steps; j) i: p‐toluenesulfonic acid, CH2Cl2/MeOH; ii: p‐toluenesulfonic acid, 2,2‐dimethoxypropane; iii: 1 M HCl, EtOAc, 0 °C, 75 % over three steps; k) i: Tf2O, pyridine, CH2Cl2, −40 °C; ii: nBuLi, di‐tert‐butyl methylphosphonate, THF, −70 to −50 °C, 72 % over two steps; l) i: methyl acrylate, CuI, Grubbs 2nd‐gen. catalyst, 1,2‐dichloroethene (DCE), 60 °C; ii: NaBH4, RuCl3, MeOH, DCE, 45 °C, 72 % over two steps; m) LiOH, THF/H2O, quant; n) Fmoc‐l‐Lys‐OMe, HCTU, DIPEA, DMF, 86 %; o) LiOH, THF/H2O, 0 °C, 80 %.
