Abstract
The polio endgame remains complicated, with many questions about future polio vaccines and national immunization policies. We simulated possible future poliovirus vaccine routine immunization policies for countries stratified by World Bank Income Levels and estimated the expected costs and cases using an updated integrated dynamic poliovirus transmission, stochastic risk, and economic model. We consider two reference cases scenarios: one that achieves the eradication of all wild polioviruses (WPVs) by 2023 and one in which serotype 1 WPV (WPV1) transmission continues. The results show that the addition of inactivated poliovirus vaccine (IPV) to routine immunization in all countries substantially increased the expected costs of the polio endgame, without substantially increasing its expected health or economic benefits. Adding a second dose of IPV to the routine immunization schedules of countries that currently include a single IPV dose further increases costs and does not appear economically justified in the reference case that does not stop WPV transmission. For the reference case that includes all WPV eradication, adding a second IPV dose at the time of successful OPV cessation represents a cost-effective option. The risks and costs of needing to restart oral poliovirus vaccine (OPV) use change the economics of the polio endgame, although the time horizon used for modeling impacts the overall economic results. National health leaders will want to consider the expected health and economic net benefits of their national polio vaccine strategies recognizing that preferred strategies may differ.
Keywords: polio, eradication, dynamic modeling, health economics
1. INTRODUCTION
The Global Polio Eradication Initiative (GPEI) continues to extend its timeline, and the costs of polio eradication continue to rise (Thompson & Kalkowska, 2020b, 2020c). A recent prospective economic analysis suggested lower costs for polio eradication followed by 2 doses of inactivated poliovirus vaccine (IPV) than for continued permanent very high control with global use of both oral poliovirus vaccine (OPV) and 2 doses of IPV in perpetuity (Zimmermann, Hagedorn, & Lyons, 2020). However, that analysis (Zimmermann et al., 2020) focused on the estimated external financial needs of the GPEI (i.e., not total costs), and it did not stratify countries by income level or account for the numerous potential poliovirus vaccines available prospectively (Thompson & Kalkowska, 2020a). As of early 2020 and prior to the COVID-19 pandemic, WPV1 continues to circulate, the GPEI is not on track to achieve WPV1 eradication (Kalkowska, Wassilak, Cochi, Pallansch, & Thompson, 2020), and the globally-coordinated cessation of serotype 2 OPV (OPV2) that occurred in mid-2016 remains off track (Kalkowska, Pallansch, Cochi, et al., 2020). National health leaders face a number of difficult choices about current and future polio vaccine use with increasing vaccine costs (Thompson & Kalkowska, 2020a). They will likely want to consider the insights from national health economic analyses (like some studies published prior to the global introduction of IPV into all countries (Griffiths, Botham, & Schoub, 2006; Mascarenas, Salinas, Tasset-Tisseau, Mascarenas, & Khan, 2005)) and consider their national risks.
As of 2016, all countries began to include at least one dose of injectable IPV into their national immunization programs in anticipation of the successful eradication of all wild polioviruses (WPVs) and globally coordinated cessation of all OPV use (Kalkowska, Wassilak, et al., 2020; Thompson & Kalkowska, 2020a). Some relatively high-income countries already use IPV exclusively for routine immunization (RI). Most middle-income countries use IPV either in a sequential schedule (i.e., IPV followed by OPV or IPV/OPV) or when delivering a single dose of IPV at the same time as the third non-birth OPV dose (i.e., OPV+IPV). Finally, relatively lower-income countries deliver a single dose of IPV at the same time as the third non-birth OPV dose (i.e., OPV+IPV), for which external financial support from the GPEI subsidized IPV introduction.
Prior economic analyses explored the health and economic costs of prospective poliovirus vaccine policies for the polio endgame (Bart, Foulds, & Patriarca, 1996; Duintjer Tebbens et al., 2011; Duintjer Tebbens, Pallansch, Wassalik, Cochi, & Thompson, 2015; Duintjer Tebbens & Thompson, 2016, 2017; Khan & Ehreth, 2003; Thompson & Duintjer Tebbens, 2015; Thompson et al., 2008; Zimmermann et al., 2020). For example, consistent with the 2013–2018 Strategic Plan (World Health Organization Global Polio Eradication Initiative, 2013), our prior health and economic analysis included expectations of: (1) eradication of all WPV serotypes by 2016, (2) cessation of all OPV use by mid-2019, (3) maintenance of high population immunity prior to effective globally-coordinated OPV cessation (minimal cVDPV risks), (4) highly-effective post-OPV cessation risk management, and (5) the introduction of one dose of IPV into RI in all countries by 2015 (Duintjer Tebbens et al., 2015; Thompson & Kalkowska, 2020b). However, delays in achieving eradication (Kalkowska, Wassilak, et al., 2020), challenges with OPV2 cessation (Kalkowska, Pallansch, Cochi, et al., 2020), increases in IPV costs (Thompson & Kalkowska, 2020a), and other changes that differ from prior analyses (Bart et al., 1996; Duintjer Tebbens et al., 2011; Duintjer Tebbens et al., 2015; Duintjer Tebbens & Thompson, 2016, 2017; Khan & Ehreth, 2003; Thompson & Duintjer Tebbens, 2015; Thompson et al., 2008; Zimmermann et al., 2020) motivate this updated health economic analysis of prospective polio vaccine policies.
2. METHODS
We use updated cost inputs (Thompson & Kalkowska, 2020a) in an updated global model (Kalkowska, Wassilak, et al., 2020) to characterize the expected vaccine costs for RI for two reference cases (RCs). The RCs include a very high control scenario that represents our characterization of the GPEI path as of early 2020 (prior to the COVID-19 pandemic) (RC2), an alternative eradication scenario (RC2*) (Kalkowska, Pallansch, Cochi, et al., 2020; Kalkowska & Thompson, 2020b), and several alternative vaccine policies for the time horizon of 2019–2029. Since the updated global model (Kalkowska, Wassilak, et al., 2020) does not anticipate eradication of serotype 1 WPV (WPV1) or subsequent globally-coordinated cessation of bivalent OPV (bOPV, containing OPV for serotypes 1 and 3), the RC2 scenario includes ongoing use of bOPV and at least 1 dose of IPV in perpetuity in OPV-using countries (Kalkowska, Pallansch, Cochi, et al., 2020). In contrast, RC2* (Kalkowska & Thompson, 2020b) achieves eradication of WPV1 before 2023 and implements bOPV cessation on January 1, 2025, at which time countries add a dose of IPV to their RI schedules (Kalkowska, Pallansch, Cochi, et al., 2020). Unlike a recent prospective economic analysis (Zimmermann et al., 2020) that assumed that all countries would adopt a minimum of 2 doses of IPV in their RI schedules, we allow for alternative policies that include a minimum of 0, 1, or 2 doses of IPV delivered in different vaccine formulations, for which we consider differences in the costs and benefits.
We use a time horizon of 2019–2029 for this analysis of prospective polio immunization policies to facilitate consistency with prior modeling (Kalkowska, Pallansch, Cochi, et al., 2020; Kalkowska & Thompson, 2020b; Kalkowska, Wassilak, et al., 2020). The updated integrated model (Kalkowska, Wassilak, et al., 2020) builds on a previously developed differential equation-based poliovirus transmission and OPV evolution model that included generic model inputs (Duintjer Tebbens et al., 2014; Duintjer Tebbens, Pallansch, Kalkowska, et al., 2013) developed following expert review (Duintjer Tebbens, Pallansch, et al., 2013a; Duintjer Tebbens, Pallansch, Kim, et al., 2013) and elicitation processes (Duintjer Tebbens, Pallansch, et al., 2013b), which supported a prior integrated dynamic poliovirus transmission, stochastic risk, and economic model (Duintjer Tebbens et al., 2015). To capture some of the heterogeneity that exists between countries, the model stratifies countries into blocks of approximately 107 million people each assigned to 2019 World Bank income levels (WBILs) (World Bank, 2019): 6 low-income (LI), 28 lower middle-income (LMI), 27 upper middle-income (UMI), and 11 high-income (HI) blocks (Kalkowska, Wassilak, et al., 2020). We assume that this stratification helps to represent the different conditions, costs, values, and preferences at the global level. We use the health economic modeling inputs and methods based on updated cost and valuation assumptions, and report cost estimates as 2019 net present values, using 2019 US dollars (US$2019) by WBIL (Thompson & Kalkowska, 2020a). Although we explore different vaccine policies, we recognize that countries can always do more than the minimum recommended policy (Thompson & Duintjer Tebbens, 2012). In this regard, we assume that only LI and LMI countries that currently use OPV+IPV would opt for the minimum policies, while UMI and HI will use only IPV with a minimum of 3 doses after cessation of the last OPV serotype, with many of these countries already using or likely to adopt a 4-dose schedule using an IPV-containing combination vaccine.
Table 1 summarizes the different alternative prospective vaccine policy options that we considered and compared either to RC2 (i.e., control scenarios) or RC2* (i.e., eradication scenarios). For HI and UMI blocks that use IPV-only or IPV/OPV RI schedules, we do not include them in Table 1 because these do not vary over the alternative scenarios (i.e., we assume that countries in these income levels will not change their polio vaccine strategy). The top of the Table 1 shows changes for the LI and LMI countries that use OPV+IPV schedules that we compare to RC2. Specifically, we consider an alternative policy in which countries that currently use 1 dose of IPV decide to revert to OPV-only (i.e., returning to zero doses of IPV on January 1, 2024 with or without planned, preventive SIAs (pSIAs) and they introduce trivalent (tOPV) into RI at that time, i.e., “tOPVRISIA” and “tOPVRI”). These options represent a return to control with tOPV only, similar to the scenarios considered as the RC in some historical analyses (Duintjer Tebbens et al., 2015; Thompson & Duintjer Tebbens, 2007; Thompson et al., 2008). We also consider the option of these countries going to 3 doses of bivalent OPV (bOPV) plus 2 doses of IPV in their national immunization schedules on January 1, 2025 (i.e., 2IPV2025), which represents an addition of 1 dose of IPV compared to their current RI schedules and reflects different timing for IPV introduction. The 2-IPV dose schedule matches the permanent control strategy modeled by an independent cost analysis (Zimmermann et al., 2020). Given actual experience with IPV, we assume that 3 countries (i.e., Sri Lanka, India, and Bangladesh) will choose to continue to use 2 doses of fractional IPV in their immunization schedules, and we assume that all other countries use 2 full IPV doses for these scenarios.
Table 1:
Prospective global policy options considered for the economic analysis compared to reference cases RC2 and RC2*
| Policy name | Description | LI/LMI countries using OPV+IPV at T0 |
|---|---|---|
| Control scenarios | ||
| RC2 | 1 dose of IPV in 2019–2029 | 2019–2029 OPV+IPV |
| tOPVRISIA | 1 dose of IPV in 2019–2023 followed by tOPV use only from January 1, 2024 with pSIAs | 2019–2023 OPV+IPV 2024–2029 OPV-only |
| tOPVRI | 1 dose of IPV in 2019–2023 followed by tOPV use only from January 1, 2024 without pSIAs | 2019–2023 OPV+IPV 2024–2029 OPV-only |
| 2IPV2025 | 1 dose of IPV in 2019–2024 followed by 2 doses of IPV from January 1, 2025 | 2019–2024 OPV+IPV 2025–2029 OPV+2IPV |
| RC2noRestarts | 1 dose of IPV in 2019–2029 | 2019–2029 OPV+IPV |
| Eradication scenarios | ||
| RC2* | 1 dose of IPV in 2019–2024 followed by 2 doses of IPV from January 1, 2025 | 2019–2024 OPV+IPV 2025–2029 IPV/IPV |
| 1IPV2025 | 1 dose of IPV in 2019–2029 | 2019–2024 OPV+IPV 2025–2029 IPV |
Abbreviations: HI, high-income; IPV, inactivate poliovirus vaccine; LMI, lower middle-income; LI, low-income; OPV, oral poliovirus vaccine; OPV+IPV, routine immunization schedule that delivers IPV with the third OPV dose; RC, reference case; RC2, control reference case; RC2*, WPV1 eradication reference case; T0, beginning of analytical time horizon (i.e., January 1, 2019); Tend, end of analytical time horizon (i.e., December 31, 2029); tOPV, trivalent OPV; tOPVRISIA, 1 dose of IPV in 2019–2023 followed by tOPV use only from January 1, 2024 with pSIAs; tOPVRI, 1 dose of IPV in 2019–2023 followed by tOPV use only from January 1, 2024 without pSIAs; VAPP, vaccine-associated paralytic polio; VDPV, vaccine-derived poliovirus; WPV1, serotype 1 wild poliovirus; 1IPV2025, 1 dose of IPV in 2019–2029; 2IPV2025, 1 dose of IPV in 2019–2024 followed by 2 doses of IPV from January 1, 2025
The model includes a relatively high probability of OPV2 restart in RI during the time horizon (Kalkowska, Pallansch, Cochi, et al., 2020), and in the event of an OPV restart, the model includes those doses. Although the Global Certification Commission certified the eradication of serotype 3 WPV (WPV3) in October 2019, we do not consider the potential switch from bOPV to mOPV1 because the GPEI partners have chosen not to pursue it, although this remains a possible option (Kalkowska & Thompson, 2020a). The probabilities and nature of OPV restarts differ for RC2 and RC2*. For RC2, only OPV2 restart may occur since bOPV use remains in RI, while for RC2*, restart of any OPV may occur given bOPV cessation in 2025. In addition, although we generally assume that OPV restart would occur, we run one alternative scenario for RC2 for which we set the OPV restart threshold to a level such that no OPV restarts trigger during the model time horizon (“RC2noRestarts”).
The bottom part of Table 1 shows the changes for the LI and LMI countries that use OPV+IPV schedules that we compare to RC2*. If successful eradication of WPV1 occurs and countries globally coordinate the cessation of all use of OPV-containing vaccines in RI, as in RC2* (Kalkowska & Thompson, 2020b), then we assume that these countries introduce a second IPV dose starting in January 1, 2025 (i.e., at the time of bOPV cessation) and continue using 2 IPV doses throughout the time horizon. We compare this RC2* scenario to the alternative of continued use of 1 IPV dose in RI through 2029 (i.e., “1IPV2025”), instead of going to 2 IPV doses. For RC2*, the eradication scenarios include OPV restarts.
We use the updated global model (Kalkowska, Wassilak, et al., 2020) to integrate population and coverage estimates to support cost estimation for the immunization options. Thus, for the RC2, RC2*, and each alternative scenario, we estimate the total number of doses of each type of vaccine purchased, delivered, and wasted in each income level per year, then multiply these by the appropriate costs for those vaccines. The framing of this analysis on vaccine costs excludes the consideration of global programmatic or other costs of polio eradication (e.g., surveillance, technical assistance, social mobilization, etc.) that could differ some for the eradication scenarios compared to control scenarios (Thompson & Kalkowska, 2020c). For this analysis of the eradiation scenarios, we focus on the relevant differences in these costs for the two scenarios and ignore the costs that would apply to both. Thus, since global programmatic costs include the purchase of vaccines for stockpiles to support OPV cessation, for the eradication scenarios we include the costs of purchasing vaccines for outbreak response stockpiles. Specifically, for the eradication scenarios, we include costs for 500 million doses each of monovalent OPV (mOPV) for serotype 1 (mOPV1) and serotype 3 (mOPV3) spread over 2023–2024 in anticipation of bOPV cessation, and 1 billion doses of IPV spread over 2028–2029 in anticipation of the end of mOPV use for outbreak response. For this analysis, we also recognize the need to include a cost premium (Ozawa, Yemeke, & Thompson, 2018) to increase coverage as required to move from RC2 to RC2* in addition to the additional vaccine doses purchased and delivered. Thus, for this analysis, we assume a one-time cost of $50 million required in 2020 as a vaccine administration cost premium required to increase coverage for RC2* compared to RC2 that we assign to the LMI (Thompson & Kalkowska, 2020c).
We calculate incremental economic outcomes using the incremental cost-effectiveness ratios (ICERs) in US$2019 per polio case and US$2019 per disability-adjusted life-year (DALY) reported by WBIL and the incremental net benefits (INBs) in US$2019 reported by income level and as a global aggregate. We label ICERs with negative incremental costs and negative prevented cases as “cost-saving, life-costing” (CSLC), ICERs with negative incremental costs but positive prevented cases as “cost-saving, life-saving” (CSLS), and ICERs with positive incremental costs but negative prevented cases as “dominated.” We explore the implications on ICER thresholds of using applying WBIL-adjusted multipliers of 0.2, 0.2, and 0.6 for LI, LMI, and UMI, respectively, to estimate health opportunity costs by WBIL as a function of population-weighted GNI per capita (Ochalek, Claxton, Lomas, & Thompson, 2020), similar to a recent analysis (Thompson & Kalkowska, 2020c). With our economic analysis framed according to WBIL, for INB estimation we use the same methods as other economic analyses and assume a societal willingness to pay equal to the population-weighted GNI per capita (by WBIL) per DALY saved (Thompson & Kalkowska, 2020c).
We code the model using the general-purpose programming language JAVA™ and the integrated development environment Eclipse™. We run stochastic simulations on the Amazon Elastic Compute Cloud (Amazon EC2), using 100 stochastic iterations for each scenario for the time horizon of 2019–2029. While this analysis applies to the situation that existed in early 2020, we emphasize that the COVID-19 pandemic may change both the epidemiological and economic situations and the availability of vaccine supplies, such that future studies will need to reassess the prospective options as they evolve.
3. RESULTS
Although we focus on vaccine costs, we consider the implications of each prospective immunization strategy with respect to expected polio cases to facilitate characterization of health-related costs and total costs. Fig. 1 shows the expected polio cases based on 100 stochastic iterations of the model for the control scenarios by WBIL: (a) LI, (b) LMI, (c) UMI, and (d) all countries (i.e., global). Consistent with most of the population in the LMI blocks, most cases occur in LMI countries (Fig. 1(b)). Fig. 1(b) also shows the tOPVRISIA and tOPVRI scenarios lead to an increase in expected incidence in the short term due to higher incidence associated with outbreaks in India, Nigeria, Pakistan and other countries because the reintroduction of tOPV leads to relatively lower serotype-specific take rates for serotypes 1 and 3 despite ongoing WPV1 transmission. Fig. 1(b) shows higher incidence for the tOPVRI scenario compared to the tOPVRISIA scenario due to lack of pSIAs and the ability of pSIAs to provide additional doses that increase population immunity to transmission for all 3 serotypes. The number of cases for the tOPVRISIA scenario declines over time compared to the first outbreaks in 2024 due to the gradual increase in population immunity to transmission for all 3 serotypes, which leads to a decrease in overall incidence with smaller periodic outbreaks attributable to the dynamics of slow build-up of susceptibility. In contrast, for the tOPVRI scenario the combination of low RI coverage and no pSIAs leads to greater incidence (i.e., the increase in WPV1 cases in the blocks representing endemic countries, build-up of susceptibility, and more explosive periodic cVDPV outbreaks of all serotypes elsewhere). For the 2IPV2025 scenario, Fig. 1(a), Fig. 1(b), and Fig. 1(c) show a decrease in expected cases compared to RC2 due to the additional IPV dose in all former OPV+IPV blocks, but not to the levels achieved by tOPVRISIA. The introduction of a second dose of IPV in 2025 in the 2IPV2025 scenario reduces the expected cases observed during the time horizon, which delays the timing of some OPV2 restarts beyond the end of the time horizon. For the RC2noRestarts scenario, Fig. 1(d) shows a decrease in cases from 2025–2028, followed by an increase in 2029 compared to RC2, caused by increase in cases in LI countries (Fig. 1(a)) and decrease in cases in LMI and UMI countries (Fig. 1(b) and Fig. 1(c)) due to lack of OPV2 restart. The effect of the censored time horizon (i.e., ending the simulation in 2029) artificially reduces the modeled impacts of restarting or not restarting OPV. The low RI coverage in many countries that inhibit the ability of OPV use to achieve eradication also substantially diminishes the benefits of IPV, which is more expensive, more difficult to deliver, and less effective at stopping transmission than OPV. Consistent with the assumption that IPV-only using countries will do more than the minimum, maintain high coverage, and not tolerate cases, the model estimates no expected cases for the scenarios for these countries (not shown). As suggested in Fig. 1(d), none of the options leads to zero cases over the time horizon, and very high control with tOPV only used in both RI and SIAs as the minimum policy offers the lowest expected cases over the time horizon.
Fig. 1:
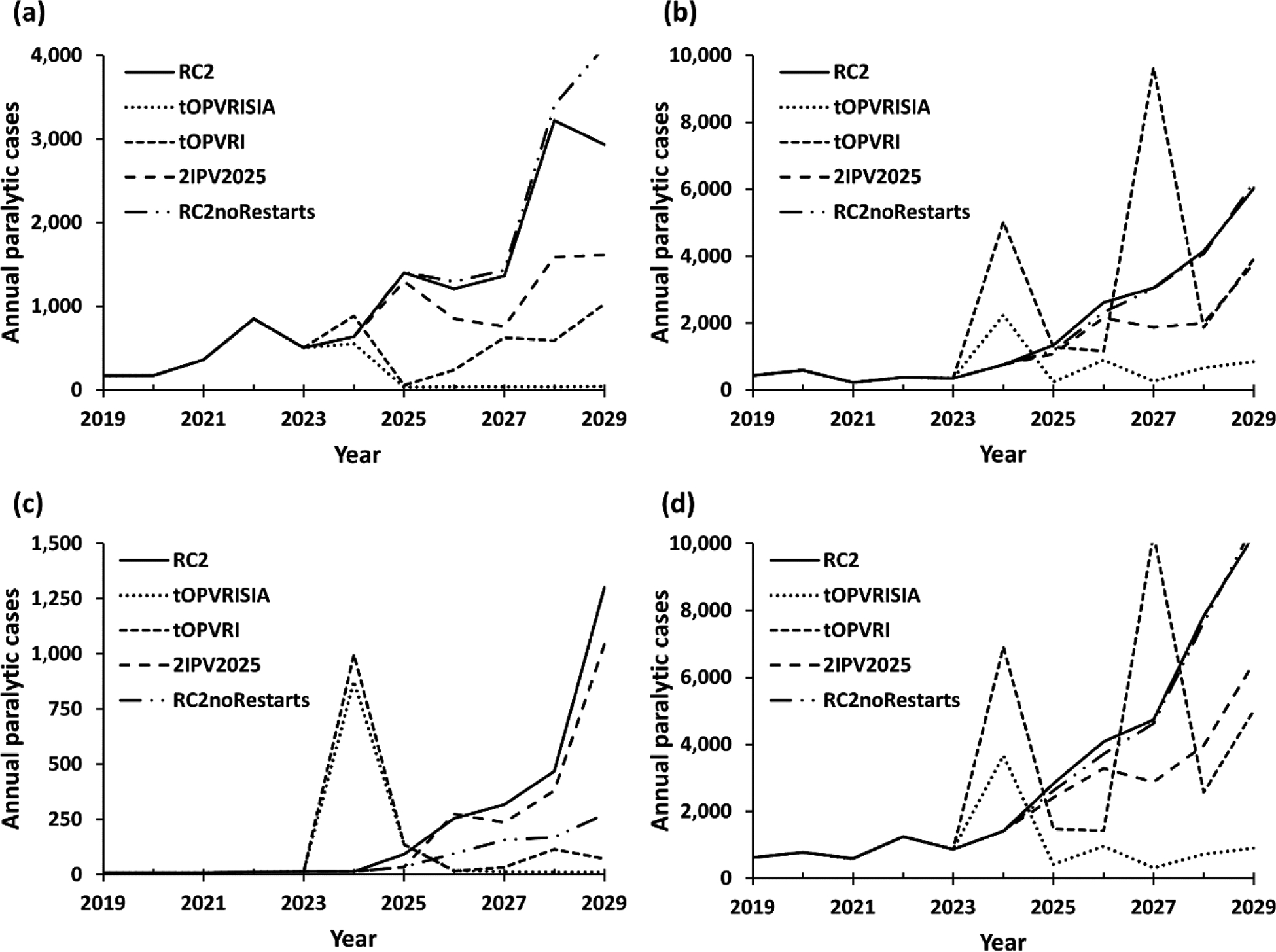
Estimated incidence for the control scenarios in the model by World Bank Income Levels and globally by year for (a) low, (b) lower middle, (c) upper middle, and (d) all countries
Note: The scales on the y-axes differ across panels
Abbreviations RC2, control reference case; RC2noRestarts, RC2 without restart option; tOPVRISIA, 1 dose of IPV in 2019–2023 followed by tOPV use only from January 1, 2024 with pSIAs; tOPVRI, 1 dose of IPV in 2019–2023 followed by tOPV use only from January 1, 2024 without pSIAs; US$2019, 2019 US dollars; 2IPV2025, 1 dose of IPV in 2019–2024 followed by 2 doses of IPV from January 1, 2025.
Fig. 2 shows the expected polio cases based on 100 stochastic iterations of the model for the eradication scenarios by WBIL: (a) LI, (b) LMI, (c) UMI, and (d) all countries (i.e., global). Due to cases that already occurred in 2019 and 2020 that suggest ongoing transmission and the high probability of OPV restarts, the eradication scenarios still show substantial numbers of cases for 2019–2029. Fig. 2(a) and 2(b) show higher incidence for the 1IPV2025 scenario in LI and LMI countries as a result of lower IPV use (i.e., 1 IPV dose less) and therefore more cases and restarts during the time horizon. Fig. 2(c) shows no difference in UMI (similar to HI, not shown) because all blocks in those income levels use IPV-only at the time of all OPV cessation on January 1, 2025 in both RC2* and 1IPV2025 scenarios. Overall, the significant risk of OPV restarts emerges as a driver of cases, even with successful eradication of WPV1, which we note with RC2* occurs with insufficient global population immunity to transmission for prevent OPV restarts.
Fig. 2:
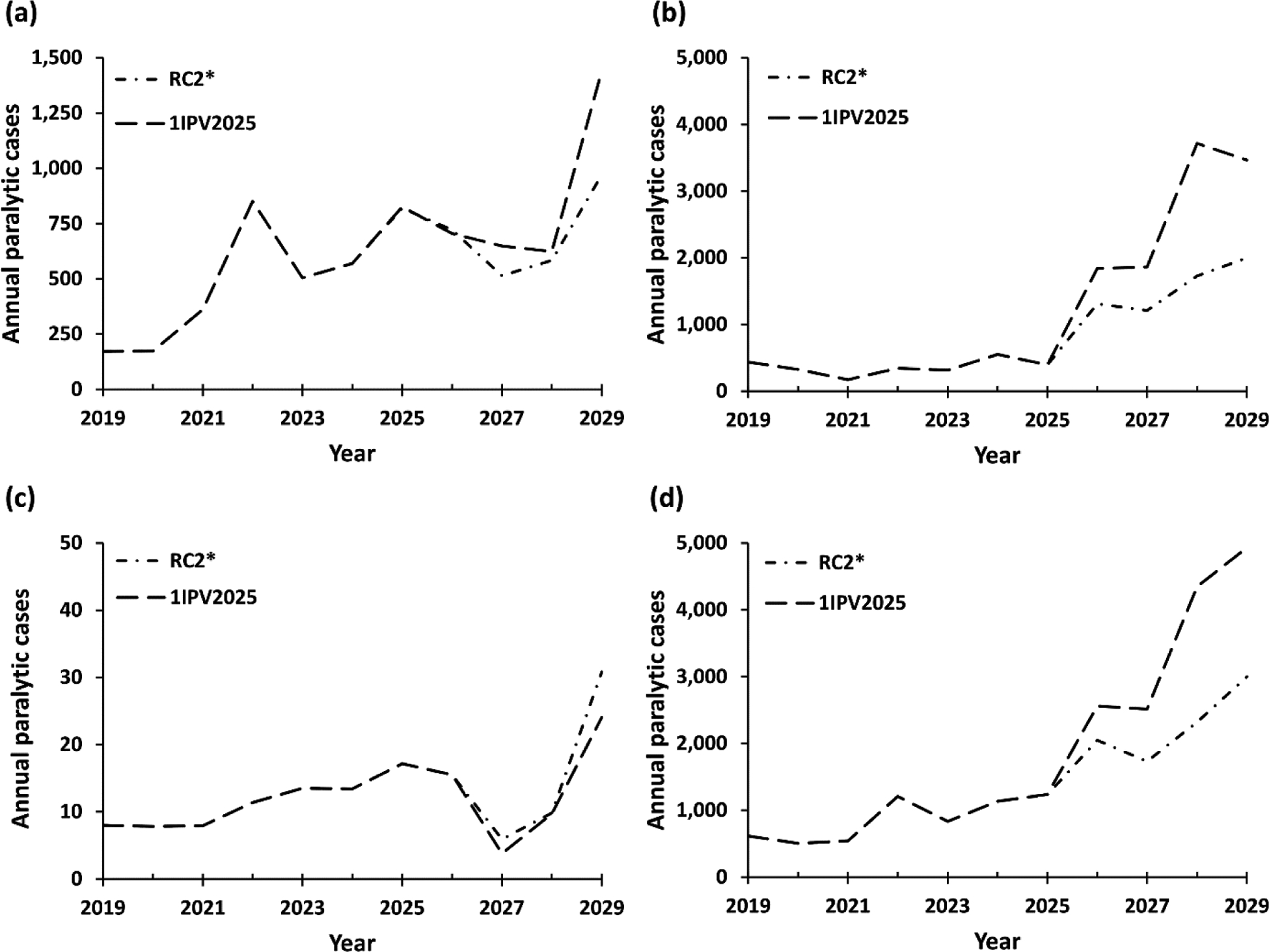
Estimated incidence for the eradication scenarios in the model by World Bank Income Levels and globally by year for (a) low, (b) lower middle, (c) upper middle, and (d) all countries
Note: The scales on the y-axes differ across panels
Abbreviations RC2*, WPV1 eradication reference case; 1IPV2025, 1 dose of IPV in 2019–2029
Table 2 summarizes the estimated number of serotype specific OPV restarts triggered within the analytical time horizon and the expected number of polio cases for different global policy options. As shown in Table 2, all of the scenarios include WPV1 cases for 2019–2021. The control scenarios in Table 2 include more WPV1 cases than the eradication scenarios, consistent with ongoing transmission of WPV1 for the control scenarios. Since both tOPVRISIA and tOPVRI represent control scenarios that end the pursuit of OPV cessation and return to the use of tOPV, Table 2 does not report any restarts triggered for those scenarios. Both scenarios that return to tOPV reduce the estimated expected total number of polio cases compared to RC2, with tOPVRISIA leading to the fewest cases overall among all considered options, while tOPVRI leads to the most WPV1 cases of all options due to the lack of pSIAs (Table 2). Since the RC2noRestarts scenario does not allow for any OPV restarts, Table 2 does not report any restarts triggered for that scenario. Table 2 shows higher estimated expected numbers of cVDPV2 cases for the RC2noRestarts scenario compared to RC2, but slightly lower estimated expected total polio cases overall due to the lack of serotype 2 containing OPV in RI that would help manage cVDPV2 outbreaks, but which also lead to circulation of lower reversion stage OPV2 viruses.
Table 2:
Expected OPV restart probabilities by serotype and expect cases by strain for different policy options (2019–2029)
| Scenario | Restarts triggered | Estimated expected cases | ||||||
|---|---|---|---|---|---|---|---|---|
| OPV1 | OPV2 | OPV3 | WPV1 | cVDPV1 | cVDPV2 | cVDPV3 | Total* | |
| Control scenarios | ||||||||
| RC2 | 0% | 89% | 0% | 1,407 | 28 | 26,226 | 0 | 35,242 |
| tOPVRISIA | 0% | 0% | 0% | 2,510 | 380 | 2,586 | 87 | 11,033 |
| tOPVRI | 0% | 0% | 0% | 6,757 | 9,922 | 2,735 | 1,185 | 31,758 |
| 2IPV2025 | 0% | 81% | 0% | 1,266 | 0 | 16,761 | 0 | 24,464 |
| RC2noRestarts | 0% | 0% | 0% | 1,382 | 0 | 30,340 | 0 | 34,668 |
| Eradication scenarios | ||||||||
| RC2* | 20% | 44% | 0% | 324** | 3,256 | 6,292 | 32 | 15,168 |
| 1IPV2025 | 57% | 49% | 0% | 327** | 7,398 | 6,367 | 44 | 20,428 |
Abbreviations: cVDPV#, circulating vaccine-derived poliovirus of serotype #; IPV, inactivated poliovirus vaccine; OPV, oral poliovirus vaccine; OPV#, OPV containing serotypes #; RC2, control reference case; RC2noRestarts, RC2 without restart option; RC2*, WPV1 eradication reference case; T0, beginning of analytical time horizon (i.e., January 1, 2019); Tend, end of analytical time horizon (i.e., December 31, 2029); tOPV, trivalent OPV; tOPVRISIA, 1 dose of IPV in 2019–2023 followed by tOPV use only from January 1, 2024 with pSIAs; tOPVRI, 1 dose of IPV in 2019–2023 followed by tOPV use only from January 1, 2024 without pSIAs; VAPP, vaccine-associated paralytic polio; VDPV, vaccine-derived poliovirus; WPV1, serotype 1 wild poliovirus; 1IPV2025, 1 dose of IPV in 2019–2029; 2IPV2025, 1 dose of IPV in 2019–2024 followed by 2 doses of IPV from January 1, 2025
Note:
includes all cases (i.e., totals from all infections with live polioviruses, including WPV, VDPVs, and VAPP, with VDPVs including cases associated with OPV-related viruses and including cases associated with containment breaches)
WPV1 cases occurring in 2019–2022 (before WPV1 eradication for the eradication scenarios)
Fig. 3 shows the expected vaccine costs by income level over time for all of the control and eradication scenarios by WBIL: (a) LI, (b) LMI, (c) UMI, and (d) LI+LMI+UMI (excluding HI, because the costs for HI countries do not vary). The costs of the scenarios include RI and SIA costs and vary over time (Fig. 3), with the control scenarios involving tOPV (i.e., tOPVRISIA or tOPVRI) showing relatively higher expected costs earlier and lower expected costs later, while the RC2noRestarts scenario shows relatively lower expected cost earlier (no additional cost of OPV2 restarts in RI) and higher expected costs later (more oSIA related costs).
Fig. 3:
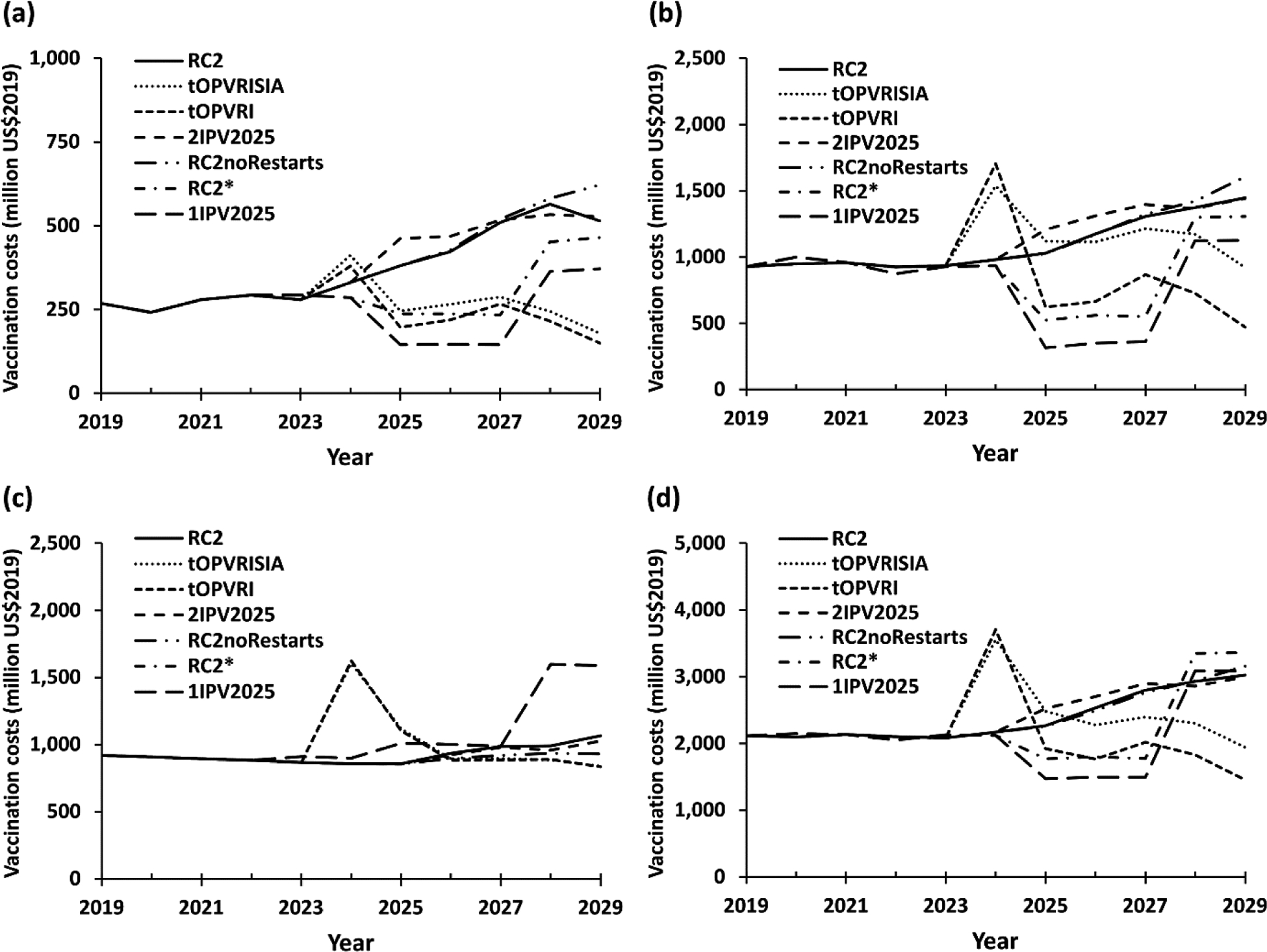
Vaccination cost estimates (US$2019) for the different scenarios in the model by World Bank Income Levels and globally by year for (a) low, (b) lower middle, (c) upper middle, and (d) all countries
Note: The scales on the y-axes differ across panels
Abbreviations RC2, control reference case; RC2noRestarts, RC2 without restart option; RC2*, WPV1 eradication reference case; tOPVRISIA, 1 dose of IPV in 2019–2023 followed by tOPV use only from January 1, 2024 with pSIAs; tOPVRI, 1 dose of IPV in 2019–2023 followed by tOPV use only from January 1, 2024 without pSIAs; US$2019, 2019 US dollars; 1IPV2025, 1 dose of IPV in 2019–2029; 2IPV2025, 1 dose of IPV in 2019–2024 followed by 2 doses of IPV from January 1, 2022.
Table 3 summarizes the results of the incremental economic analyses for alternative global policy options compared to their respective RCs by WBIL and the aggregated INB over the 11-year time horizon. Table 3 separately shows (a) the control scenarios for comparison to RC2 and (b) the eradication scenarios for comparison to RC2*, and we emphasize that in the context of this incremental analysis, all of the other programmatic costs that we ignored cancel out in the relevant comparisons. Compared to the current modeled GPEI path (i.e., RC2), Table 3(a) shows that shifting to tOPVRISIA or tOPVRI lead to expected INBs of 1.5 and 3.1 billion US$2019, respectively, increasing the minimum of one IPV dose policy to 2 IPV doses from 2025 on (i..e, 2IPV2025) decreases the expected INB by 0.1 billion US$2019, whereas continuing RC2 without restarting OPV2 use (i.e. RC2noRestarts) leads to expected INB of 0.2 billion US$2019. The ICER results suggest that returning to tOPV use represents a CSLS option for LI countries but does not represent a cost-effective option for UMI countries. For UMI countries, the use of 2 IPV doses represents a CSLS compared to RC2. For LMI, the ICER results shows the importance of pSIAs for the scenarios that switch to tOPV. As shown in Table 2, shifting to 2IPV2025 decreases the probability of triggering an OPV2 restart by 8% during the time horizon (compared to RC2, Table 2), but this does not offset the overall decline in INBs. Moreover, the RC2noRestarts option represents a CSLS option for UMI countries.
Table 3:
Incremental economic analysis estimates (US$2019) for different immunization options for different policy options by World Bank Income Levels (2019–2029)
| Vaccine policy | Incremental financial costs (US$2019 billions) | Paralytic polio cases prevented | ICER comparator threshold ($/DALY) | Incremental cost-effectiveness ratio (ICER) | Incremental net benefits (INBs) (US$201 | ||
|---|---|---|---|---|---|---|---|
| Health opportunity cost approximation | Assuming 1 GNI per capita per DALY | Per polio case (US$2019/case) | Per polio case (US$2019/DALY) | ||||
| tOPVRISIA vs. RC2 | |||||||
| LI | −1.1 | 10,024 | 173 | 866 | CSLS | CSLS | 1.2 |
| LMI | −0.2 | 12,802 | 462 | 2,310 | CSLS | CSLS | 0.6 |
| UMI | 0.5 | 1,383 | 5,484 | 9,140 | 321,624 | 321,624 | −0.3 |
| Total | −0.8 | 24,209 | NA | NA | NA | NA | 1.5 |
| tOPVRI vs. RC2 | |||||||
| LI | −1.3 | 7,341 | 173 | 866 | CSLS | CSLS | 1.4 |
| LMI | −2.3 | −4,935 | 462 | 2,310 | CSLC | CSLC | 2.1 |
| UMI | 0.5 | 1,077 | 5,484 | 9,140 | 417,289 | 417,289 | −0.4 |
| Total | −3.0 | 3,484 | NA | NA | NA | NA | 3.1 |
| 2IPV2025 vs. RC2 | |||||||
| LI | 0.1 | 4,018 | 173 | 866 | 28,564 | 28,564 | −0.1 |
| LMI | 0.4 | 6,295 | 462 | 2,310 | 55,870 | 55,870 | −0.2 |
| UMI | −0.1 | 465 | 5,484 | 9,140 | CSLS | CSLS | 0.2 |
| Total | 0.4 | 10,778 | NA | NA | NA | NA | −0.1 |
| RC2noRestarts vs. RC2 | |||||||
| LI | 0.1 | −1,513 | 173 | 866 | dominated | dominated | −0.2 |
| LMI | 0.2 | 380 | 462 | 2,310 | 557,565 | 557,565 | −0.2 |
| UMI | −0.3 | 1,707 | 5,484 | 9,140 | CSLS | CSLS | 0.6 |
| Total | 0.1 | 574 | NA | NA | NA | NA | 0.2 |
| Vaccine policy | Incremental financial costs (US$2019 billions) | Paralytic polio cases prevented | ICER comparator threshold ($/DALY) | Incremental cost-effectiveness ratio (ICER) | Incremental net benefits (INBs) (US$201 | ||
| Health opportunity cost approximation | Assuming 1 GNI per capita per DALY | Per polio case (US$2019/case) | Per polio case (US$2019/DALY) | ||||
| 1IPV2025 vs. RC2* | |||||||
| LI | −0.5 | −634 | 173 | 866 | CSLC | CSLC | 0.4 |
| LMI | −1.0 | −4,635 | 462 | 2,310 | CSLC | CSLC | 0.8 |
| UMI | −0.0 | 9 | 5,484 | 9,140 | CSLS | CSLS | 0.0 |
| Total | −1.4 | −5,260 | NA | NA | NA | NA | 1.3 |
Abbreviations CSLC, cost-saving, life-costing; CSLS, cost-saving, life-saving; cVDPV#, circulating vaccine-derived poliovirus of serotype #; DALY, disability-adjusted life-year; HI, high-income; IPV, inactivated poliovirus vaccine; LI, low-income; LMI, lower middle-income; NA, not-applicable; OPV, oral poliovirus vaccine; OPV#, OPV containing serotypes #; RC2, control reference case; RC2noRestarts, RC2 without restart option; RC2*, WPV1 eradication reference case; T0, beginning of analytical time horizon (i.e., January 1, 2019); Tend, end of analytical time horizon (i.e., December 31, 2029); tOPV, trivalent OPV; tOPVRISIA, 1 dose of IPV in 2019–2023 followed by tOPV use only from January 1, 2024 with pSIAs; tOPVRI, 1 dose of IPV in 2019–2023 followed by tOPV use only from January 1, 2024 without pSIAs; UMI, upper middle-income; US$2019, 2019 US dollars; 1IPV2025, 1 dose of IPV in 2019–2029; 2IPV2025, 1 dose of IPV in 2019–2024 followed by 2 doses of IPV from January 1, 2025.
Compared to RC2*, Table 3(b) shows that maintaining the minimum of 1 IPV dose after global OPV cessation as the minimum policy instead of introducing the second IPV dose (1IPV2025) leads to an expected 1.3 billion INBs compared to RC2*. However, as shown in Table 2, maintaining the 1IPV2025 scenario increases the probability of triggering OPV1 restarts by 37% and OPV2 restarts by 5% compared to RC2*. These results reflect the already relatively high expected risks of OPV2 restart (Kalkowska, Pallansch, Cochi, et al., 2020) and insufficient population immunity to transmission for serotype 1 in RC2* prior to bOPV cessation to prevent the development of cVDPVs. As noted elsewhere (Kalkowska & Thompson, 2020a, 2020b; Thompson & Kalkowska, 2020c), these results suggest the need to increase population immunity to transmission for serotype 1 prior to bOPV cessation in 2025 to reduce cVDPV1 risks and OPV1 restarts (Duintjer Tebbens, Hampton, & Thompson, 2018; Duintjer Tebbens et al., 2016).
Comparison of the RC2noRestarts and RC2 scenarios show the impacts of no OPV restarts on the overall economics. While OPV restarts increase immunization costs for countries in all income levels, restarting OPV prevents future cases (particularly in LI and LMI), which leads to savings on treatment costs and reductions of productivity losses. These off-setting effects may seem counter-intuitive, because the iterations with OPV restarts imply more than 5,000 cases to trigger the restart. However, the comparison without OPV2 restarts helps to demonstrate the net effects on costs and cases through the model time horizon. Use of a time horizon through 2029 censors the analysis with respect to longer time horizon effects. Notably, in the long term, the scenarios that do not include eradication of live polioviruses will continue to lead to transmission, cases, and associated treatment costs and productivity losses. Over the longer term, the scenarios with and without OPV2 restarts (i.e., RC2 compared to RC2noRestarts) would diverge more with respect to expected cases, with the number of expected cases continuing the trend reported in 2029 (i.e., higher cases for RC2noRestarts than RC2). The scenarios that include IPV will reduce the numbers of cases, thus delaying some OPV restarts, but do so at relatively high cost.
4. DISCUSSION
The high probability of OPV2 restarts for the eradication scenarios suggest that future GPEI strategies should address OPV restart risks and should consider increasing pSIAs prior to bOPV cessation (Duintjer Tebbens et al., 2018; Duintjer Tebbens et al., 2016). The high probability of OPV2 restarts for the control scenarios suggest that future GPEI strategies should address OPV restart risks and recognize the limited value of continuing IPV in OPV-using countries following the reintroduction of tOPV. The GPEI recently released a strategy for managing cVDPV2s that pose a risk for OPV2 restart (World Health Organization Global Polio Eradication Initiative, 2020), which relies heavily on planned use of a novel vaccine strain of OPV2 (nOPV2). Recent modeling suggests that nOPV2 use for outbreak response SIAs alone will not likely lead the success for the 2016 OPV2 globally-coordinated cessation (Kalkowska, Pallansch, Wilkinson, et al., 2020). Future studies will need to consider the different potential roles of novel OPV strains for all serotypes.
Even in the absence of strategies to address OPV2 restart risks, this analysis demonstrates that poliovirus vaccination for 2019–2029 will continue to cost billions of US$2019 per year, with lower costs in LI and LMI than in UMI and HI countries. Despite decades of investment in global polio eradication, the costs of polio immunization continue to increase with the adoption of more expensive vaccines and immunization strategies that increase the total numbers of doses delivered. Thus, in contrast to the experience with smallpox eradication, which led to the elimination of smallpox vaccine use and associated cost savings, global polio eradication does not appear to be heading toward the elimination of poliovirus vaccination. National immunization programs will need to budget for substantial costs for purchasing and delivering polio vaccination prospectively.
The insights of this analysis remain limited by the model structure and assumptions (see details in (Kalkowska, Wassilak, et al., 2020) and its technical appendix), and the stochastic nature of the iterations. Future studies will need to consider the impacts of the global COVID-19 pandemic on poliovirus transmission changes due to reduced mixing and reduced immunization. The model includes consideration of the risks of OPV restarts, but does not consider the impacts of any new vaccine options (e.g., a new OPV strain (Van Damme et al., 2019)) and/or GPEI strategies, and consequently future analyses will need to consider these as well. In addition, in late 2020, the GPEI and the World Health Organization (WHO) recommended that all countries include a minimum of 2 doses of IPV in their RI schedules (World Health Organization, 2020), without any consideration of the health economics of this recommendation (Thompson & Kalkowska, 2020b). The Gavi Alliance will likely support the introduction of a second dose of IPV into some national RI schedules as early as 2021, but considerable uncertainty remains about the prospective adoption of a minimum of 2 doses of IPV in RI. Future studies will need to model the impacts of this recommended change in immunization, which we expect will increase costs.
As countries plan their national budgets, our results suggest the need to plan for continued polio expenditures for polio vaccine and administration for the foreseeable future. The future of IPV use will likely depend on financing, which we expect national leaders will continue to evaluate as part of their national immunization portfolios. We hope that this analysis provides a broader perspective on the possible options for prospective use of polio vaccines.
Supplementary Material
ACKNOWLEDGMENTS
This publication was supported by Cooperative Agreement Number 5NU2RGH001913-03-00 funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
REFERENCES
- Bart K, Foulds J, & Patriarca P (1996). Global eradication of poliomyelitis: benefit-cost analysis. Bulletin of the World Health Organization, 74, 35–45. [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Hampton LM, & Thompson KM (2018). Planning for globally coordinated cessation of bivalent oral poliovirus vaccine: risks of non-synchronous cessation and unauthorized oral poliovirus vaccine use. BMC Infect Dis, 18(1), 165. doi: 10.1186/s12879-018-3074-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Hampton LM, Wassilak SGF, Pallansch MA, Cochi SL, & Thompson KM (2016). Maintenance and intensification of bivalent oral poliovirus vaccine use prior to its coordinated global cessation. Journal of Vaccines and Vaccination, 7(5), 340. doi: 10.4172/2157-7560.1000340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Kalkowska DA, Wassilak SG, Pallansch MA, Cochi SL, & Thompson KM (2014). The potential impact of expanding target age groups for polio immunization campaigns. BMC Infectious Diseases, 14, 45. doi: 10.1186/1471-2334-14-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, Halsey NA, Hovi T, Minor PD, … Thompson KM (2013a). Expert review on poliovirus immunity and transmission. Risk Analysis, 33(4), 544–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, Halsey NA, Hovi T, Minor PD, … Thompson KM (2013b). Review and assessment of poliovirus immunity and transmission: Synthesis of knowledge gaps and identification of research needs. Risk Analysis, 33(4), 606–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Cochi SL, Wassilak SGF, Linkins J, Sutter RW, … Thompson KM (2011). Economic analysis of the Global Polio Eradication Initiative. Vaccine, 29(2), 334–343. [DOI] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Kalkowska DA, Wassilak SGF, Cochi SL, & Thompson KM (2013). Characterizing poliovirus transmission and evolution: Insights from modeling experiences with wild and vaccine-related polioviruses. Risk Analysis, 23(4), 703–749. [DOI] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Kim J-H, Burns CC, Kew OM, Oberste MS, … Thompson KM (2013). Review: Oral poliovirus vaccine evolution and insights relevant to modeling the risks of circulating vaccine-derived polioviruses (cVDPVs). Risk Analysis, 23(4), 680–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Wassalik SGF, Cochi SL, & Thompson KM (2015). An economic analysis of poliovirus risk management policy options for 2013–2052. BMC Infectious Diseases, 15(389), doi: 10.1186/s12879-12015-11112-12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, & Thompson KM (2016). The potential benefits of a new poliovirus vaccine for long-term poliovirus risk management. Future Microbiology, 11(12), 1549–1561. [DOI] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, & Thompson KM (2017). Costs and benefits of including inactivated in addition to oral poliovirus vaccine in outbreak response after cessation of oral poliovirus vaccine use. Med Decis Making Policy & Practice, 2(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths UK, Botham L, & Schoub BD (2006). The cost-effectiveness of alternative polio immunization policies in South Africa. Vaccine, 24(29–30), 5670–5678. doi: 10.1016/j.vaccine.2006.05.032 [DOI] [PubMed] [Google Scholar]
- Kalkowska DA, Pallansch MA, Cochi SL, Kovacs SD, Wassilak SGF, & Thompson KM (2020). Updated characterization of post-OPV cessation risks: Lessons from 2019 serotype 2 outbreaks and implications for the probability of OPV restart. Risk Analysis, online July 6, doi: 10.1111/risa.13555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Pallansch MA, Wilkinson A, Bandyopadhyay AS, Konopka-Anstadt JL, Burns CC, … Thompson KM (2020). Updated characterization of poliovirus outbreak response strategies for 2019–2029: Impacts of the use of novel OPV2 strains. Risk Analysis, online September 16, doi: 10.1111/risa.13590 . doi:10.1111/risa.1359010.1111/risa.13622. doi: 10.1111/risa.13622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, & Thompson KM (2020a). Expected implications of globally-coordinated cessation of serotype 3 oral poliovirus vaccine (OPV) before serotype 1 OPV. Risk Analysis, online September 16, doi: 10.1111/risa.13590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, & Thompson KM (2020b). Insights from modeling preventive supplemental immunization activities as a strategy to eliminate wild poliovirus transmission in Pakistan and Afghanistan. Risk Analysis, online March 6, doi: 10.1111/risa.13471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Wassilak SGF, Cochi SL, Pallansch MA, & Thompson KM (2020). Global transmission of live polioviruses: Updated integrated dynamic modeling of the polio endgame. Risk Analysis online January 22, doi: 10.1111/risa.13447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MM, & Ehreth J (2003). Costs and benefits of polio eradication: a long-run global perspective. Vaccine, 21, 702–705. [DOI] [PubMed] [Google Scholar]
- Mascarenas A, Salinas J, Tasset-Tisseau A, Mascarenas C, & Khan MM (2005). Polio immunization policy in Mexico: economic assessment of current practice and future alternatives. Public Health, 119(6), 542–549. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15826896 [DOI] [PubMed] [Google Scholar]
- Ochalek J, Claxton K, Lomas J, & Thompson KM (2020). Valuing health outcomes: Developing better defaults based on health opportunity costs. Expert Review of Pharmacoeconomics & Outcomes Research, 10.1080/14737167.2020.1812387 [DOI] [PubMed] [Google Scholar]
- Ozawa S, Yemeke TT, & Thompson KM (2018). Systematic review of the incremental costs of interventions that increase immunization coverage. Vaccine, 36(25), 3641–3649. doi: 10.1016/j.vaccine.2018.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, & Duintjer Tebbens RJ (2007). Eradication versus control for poliomyelitis: an economic analysis. Lancet, 369(9570), 1363–1371. doi: 10.1016/s0140-6736(07)60532-7 [DOI] [PubMed] [Google Scholar]
- Thompson KM, & Duintjer Tebbens RJ (2012). Current polio global eradication and control policy options: perspectives from modeling and prerequisites for oral poliovirus vaccine cessation. Expert Review of Vaccines, 11(4), 449–459. doi: 10.1586/erv.11.195 [DOI] [PubMed] [Google Scholar]
- Thompson KM, & Duintjer Tebbens RJ (2015). Health and economic consequences of different options for timing the coordinated global cessation of the three oral poliovirus vaccine serotypes. BMC Infectious Diseases, 15(374), doi: 10.1186/s12879-12015-11113-12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, Duintjer Tebbens RJ, Pallansch MA, Kew OM, Sutter RW, Aylward RB, … Cochi SL (2008). The risks, costs, and benefits of possible future global policies for managing polioviruses. American Journal of Public Health, 98(7), 1322–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, & Kalkowska DA (2020a). Potential future use, costs, and value of poliovirus vaccines. Risk Analysis, online July 9, doi: 10.1111/risa.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, & Kalkowska DA (2020b). Reflections on modeling poliovirus transmission and the polio eradication endgame. Risk Analysis, online April 27, doi: 10.1111/risa.13484. doi: 10.1111/risa.13484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, & Kalkowska DA (2020c). An updated economic analysis of the Global Polio Eradication Initiative. Risk Analysis, 10.1111/risa.13665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme P, De Coster I, Bandyopadhyay AS, Revets H, Withanage K, De Smedt P, … Gast C (2019). The safety and immunogenicity of two novel live attenuated monovalent (serotype 2) oral poliovirus vaccines in healthy adults: a double-blind, single-centre phase 1 study. Lancet, 394(10193), 148–158. doi: 10.1016/s0140-6736(19)31279-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank. (2019). World Bank list of economies (June 2019). Retrieved from http://databank.worldbank.org/data/download/site-content/CLASS.xls
- World Health Organization. (2020). Meeting of the Strategic Advisory Group of Experts on immunization, October 2020 – conclusions and recommendations. Wkly Epidemiol Rec, 95(48), 585–608. [Google Scholar]
- World Health Organization Global Polio Eradication Initiative. (2013). Polio eradication and endgame Strategic Plan (2013–2018). Geneva; 2013. Report No: WHO/POLIO/13.02. Retrieved from http://polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_A4.pdf [Google Scholar]
- World Health Organization Global Polio Eradication Initiative. (2020). Strategy for the response to type 2 circulating vaccine-derived poliovirus 2020–2021: Addendum to the Polio eradication and endgame strategic plan (2019–2023). Geneva; 2020. Report No: WHO/POLIO/20.02. Retrieved from http://polioeradication.org/wp-content/uploads/2020/04/Strategy-for-the-response-to-type-2-circulating-Vaccine-Derived-Poliovirus-20200406.pdf [Google Scholar]
- Zimmermann M, Hagedorn B, & Lyons H (2020). Projection of costs of polio eradication compared to permanent control. Journal of Infectious Diseases, 221(4), 561–565. doi: 10.1093/infdis/jiz488 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


