Abstract
Cognitive reserve (CR) is thought to protect against the consequence of age- or disease-related structural brain changes across multiple cognitive domains. The neural basis of CR may therefore comprise a functional network that is actively involved in many different cognitive processes. To investigate the existence of such a “task-invariant” CR network, we measured functional connectivity in a cognitively normal sample between 20 and 80 years old (N = 265), both at rest and during the performance of 11 separate tasks that aim to capture four latent cognitive abilities (i.e. vocabulary, episodic memory, processing speed, and fluid reasoning). For each individual, we determined the change in functional connectivity from the resting state to each task state, which is referred to as “task potency” (Chauvin et al., 2018, 2019). Task potency was calculated for each pair among 264 nodes (Power et al., 2012) and then summarized across tasks reflecting the same cognitive ability. Subsequently, we established the correlation between task potency and IQ or education (i.e. CR factors). We identified a set of 57 pairs in which task potency showed significant correlations with IQ, but not education, across all four cognitive abilities. These pairs were included in a principal component analysis, from which we extracted the first component to obtain a latent variable reflecting task potency in this task-invariant CR network. This task potency variable was associated with better episodic memory (β = 0.19, p < .01) and fluid reasoning performance (β = 0.17, p < .01) above and beyond the effects of cortical thickness (range [absolute] β = 0.28–0.32, p < .001). Our identification of this task-invariant network contributes to a better understanding of the mechanism underlying CR, which may facilitate the development of CR-enhancing treatments. Our work also offers a useful alternative operational measure of CR for future studies.
Keywords: Cognitive aging, Cognitive reserve, Cortical thickness, IQ, fMRI
1. Introduction
Cognitive reserve (CR) describes the ability to maintain cognitive function in the presence of age- or disease-related structural brain changes (Stern, 2012). Individuals with higher educational attainment, IQ, and physical or cognitive activity, among other factors, generally have greater CR (Arenaza-Urquijo et al., 2015; Bennett et al., 2003; Groot et al., 2016; Rentz et al., 2007; Scarmeas et al., 2003, 2009; Valenzuela and Sachdev, 2006; Wilson et al., 2010, 2013). A common way to demonstrate the CR phenomenon is to show that a hypothesized CR factor or mechanism relates to better cognition after adjusting for brain structure (Stern and Habeck, 2018). Such findings have been reported for various cognitive domains, and therefore the neural basis of CR may comprise a functional network that is actively involved in many different cognitive processes. Functional magnetic resonance imaging (fMRI) has indeed provided evidence for the existence of a “task-invariant” mechanism underlying CR. Based on the comparison of BOLD activation patterns from multiple cognitive tasks, several studies identified common regions of activity across conditions (Habeck et al., 2016; Stern et al., 2008, 2018). Recruitment of these regions showed direct associations with CR factors, such as education (Habeck et al., 2016) and IQ (Stern et al., 2008, 2018). In addition, resting state fMRI, which has the advantage of providing information about the brain’s functional organization rather than regional activity, has also been used in the context of CR. These studies have suggested that CR is represented by global connectivity of specific “cognitive control” regions (Cole et al., 2012; Franzmeier et al., 2017a; Franzmeier et al., 2017b), which support cognition at a task-invariant level by mediating flexible adaptation to changing task demands.
Although there is general consensus on the existence of a link between resting state functional connectivity and cognition (van den Heuvel and Hulshoff Pol, 2010), this link is indirect in nature as these fMRI data are not acquired during task performance. In fact, several studies have shown that functional connectivity is dynamic (Hutchison et al., 2013; Gonzalez-Castillo and Bandettini, 2017) and systematic differences between rest and task states exist despite overall preservation of network topography (Braun et al., 2015; DeSalvo et al., 2014; Mennes et al., 2013). This is consistent with the idea that neural responses evoked by tasks build upon an already present functional connectivity baseline (Fox et al., 2006; Smith et al., 2009; Tavor et al., 2016). These systematic differences between resting state and task-based functional connectivity have motivated the development of novel fMRI analysis techniques, such as the “task potency” method (Chauvin et al., 2018, 2019). Task potency captures a brain region’s functional connectivity during task performance after adjusting for its resting state baseline and thus reflects connectivity changes that occur in response to an experimental condition. As the task potency method provides task-related information while also taking into account the interconnected nature of the brain, it offers an ideal approach for the identification of a network that is actively involved in multiple tasks (Chauvin et al., 2019).
In the present study, we used a technique that closely resembles the task potency method to investigate the existence of a task-invariant CR network. We used data from the Reference Ability Neural Network (RANN) study (Stern et al., 2014), in which healthy individuals across the adult age span (i.e. 20–80) underwent fMRI during rest and while performing a large set of cognitive tasks. These tasks reflect four latent cognitive abilities that capture most of the age-related variance in cognitive performance: vocabulary, episodic memory, processing speed, and fluid reasoning (Salthouse, 2005, 2009; Salthouse et al., 2008). Based on 11 RANN tasks, we calculated task potency maps for each cognitive ability and then assessed their relationship with known CR factors (i.e. education and IQ). We aimed to find a common network of connections in which task potency consistently correlated with these CR factors across all cognitive abilities. To test if the network behaved in accordance with the CR theory, we established its influence on cognition relative to the effects of brain structure. We predicted that greater expression of a CR-related task potency pattern in this network was associated with better cognitive performance after adjusting for cortical thickness.
2. Material and methods
2.1. Participants
All subjects participated in the RANN study, which was approved by the Columbia University Institutional Review Board. Individuals were recruited through random market mailing and provided written informed consent prior to participation. Subjects were required to be aged between 20 and 80 years, native English speakers, right-handed, and have at least a fourth grade reading level. Subjects were screened for MRI contraindications and hearing or visual impairment that would impede testing. Subjects were free of medical or psychiatric conditions that could affect cognition. Careful screening ensured that the elder subjects did not meet criteria for dementia or mild cognitive impairment (MCI). A score greater than 130 was required on the Mattis Dementia Rating Scale (Mattis, 1988) to ensure cognitive normalcy. Further, participants were required to have no or minimal complaints on a functional impairment questionnaire (Blessed et al., 1968). From this RANN cohort, we selected all subjects (n = 323) who completed fMRI scans during rest and while performing 11 RANN tasks (as described below, Picture Naming was not considered for analysis here). Our final sample consisted of 265 subjects, after exclusion of those with high proportions of missing or scrubbed fMRI data (see Fig. 1). Individuals who were excluded had a higher mean age and lower global mean cortical thickness; there were no other differences compared to the included sample (see Supplementary Table 1). The datasets and codes generated for this study are available on request to the corresponding author.
Fig. 1.
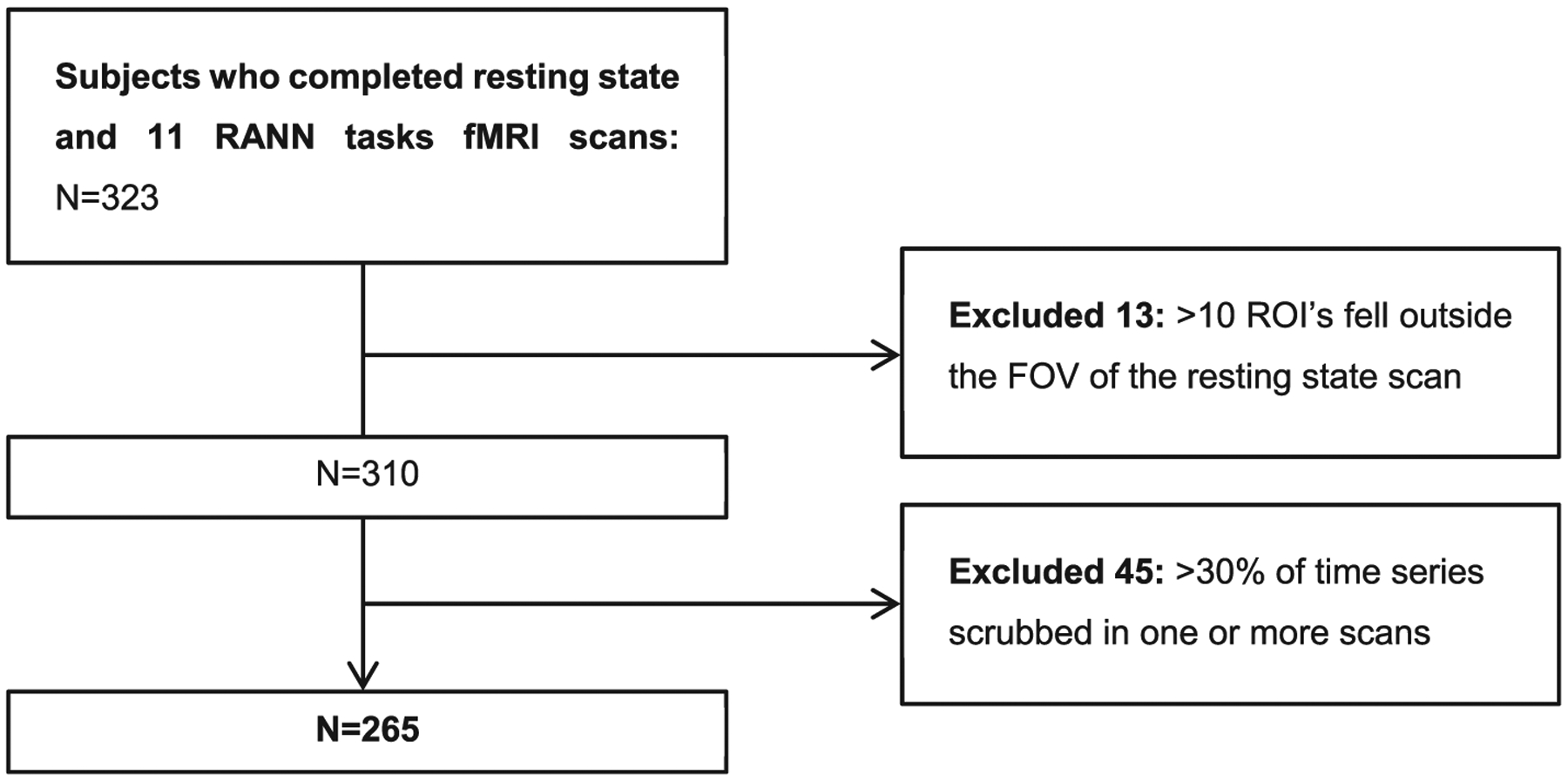
Flowchart illustrating the inclusion/exclusion of individuals in this study.
RANN = Reference Ability Neural Network study, ROI = region of interest according to Power et al. (2011), FOV = field of view.
2.2. Cognitive reserve factors
We measured education and IQ as they are both contributing factors of CR (Arenaza-Urquijo et al., 2015). Education was measured in years, and IQ was estimated based on American National Adult Reading Test (NART) scores (Grober and Sliwinski, 1991).
2.3. Cognitive assessment
Task stimuli were back-projected onto a screen located at the foot of the MRI bed using an LCD projector. Participants viewed the screen via a mirror system located in the head coil and, if needed, had vision corrected to normal using MR compatible glasses (manufactured by Safe-Vision, LLC. Webster Groves, MO). Responses were made on a LUMItouch response system (Photon Control Company). Task administration and collection of reaction time (RT) and accuracy data were controlled by EPrime running on a PC. Task onset was electronically synchronized with the MRI acquisition computer (Stern et al., 2014).
We used all tasks from the RANN study, except one (i.e. Picture Naming). Since this task requires verbal responses in the scanner, it presumably evokes functional connectivity patterns related to speech production that are not present in other tasks. Moreover, verbally responding considerably increased the amount of head movement during the scan, which may introduce task-related biases and thus further complicates the comparison with other RANN tasks. For the remaining 11 tasks, functional connectivity was computed based on the entire duration of each scan (e.g. for episodic memory tasks, both the encoding and retrieval conditions were included). We briefly describe each RANN task below, grouped by their four latent cognitive abilities; more details are provided elsewhere (Stern et al., 2014). Scores for each task were standardized using the total baseline sample of the RANN study as a reference group (N = 396), and for each cognitive ability we created a summary score by averaging z-scores from tasks within the same ability.
2.3.1. Vocabulary (VOCAB)
The primary dependent variable for all VOCAB tasks is the proportion of correct items. Antonyms (Salthouse, 1993): Participants match a given word to its antonym, or to the word most different in meaning. Synonyms (Salthouse, 1993): Subjects have to match a given word to its synonym or to the word most similar in meaning, by selecting one option from a set of other words. In both cases, the probe word is presented in all capital letters at the top of the screen, and four numbered choices are presented below.
2.3.2. Episodic memory (MEM)
The primary dependent variable for the episodic memory tests is the proportion of correctly answered questions. Logical Memory: Stories are presented on the computer screen. The subject is asked to answer detailed multiple-choice questions about the story, with four possible answer choices. Paired Associates: Pairs of words are presented, one at a time, on the screen, and subjects are instructed to remember the pairs. Following the pairs, they were given a probe word at the top of the screen and four additional word choices below. Subjects were asked to choose the word that was originally paired with the probe word. Word Order Recognition: A list of twelve words is presented one at a time on the screen, and subjects are instructed to remember the order in which the words are presented. Following the word list they are given a probe word at the top of the screen, and four additional word choices below. They are instructed to choose out of the four options the word that immediately followed the word given above.
2.3.3. Perceptual speed (SPEED)
The primary dependent variable for all SPEED tasks is reaction time. Letter Comparison (Salthouse and Babcock, 1991): In this task, two strings of letters, each consisting of three to five letters, are presented alongside one another. Subjects indicate whether the strings are the same or different using a differential button press. Pattern Comparison (Salthouse and Babcock, 1991): Two figures consisting of varying numbers of lines connecting at different angles are presented alongside one another. Subjects indicate whether the figures were the same or different by a differential button press. Digit Symbol: A code table is presented on the top of the screen, consisting of numbers one through nine, each paired with an associated symbol. Below the code table an individual number/symbol pair is presented. Subjects are asked to indicate whether the individual pair is the same as that in the code table using a differential button press. Subjects are instructed to respond as quickly and accurately as possible.
2.3.4. Fluid reasoning (FLUID)
The primary dependent variable for FLUID tasks is the proportion of correct trials completed. Matrix Reasoning [adapted from (Raven, 1962]): Subjects are given a matrix that is divided into nine cells, in which the figure in the bottom right cell is missing. Below the matrix, they are given eight figure choices, and they are instructed to evaluate which of the figures would best complete the missing cell. Paper Folding (Ekstrom et al., 1976): Subjects select a pattern of holes (from five options) that would result from a sequence of folds in a piece of paper, through which a hole is then punched. The sequence is given on the top of the screen, and the five options are given in a row below. Response consisted of pressing 1 of 5 buttons corresponding to the chosen solution. Letter Sets (Ekstrom et al., 1976): Subjects are presented with five sets of letters, where four out of the five sets have a common rule (i.e. have no vowels), with one of the sets not following this rule. Subjects are instructed to select the unique set.
2.4. MRI acquisition
All MR images were acquired on a 3.0T Philips Achieva Magnet scanner. Participants underwent two imaging sessions of approximately 2 h. Each session started with a scout, T1-weighted image to determine patient position, after which an MPRAGE scan, 11 fMRI tasks, a resting state BOLD (ranging between 5 and 9.5 min), and other imaging modalities were obtained (i.e. FLAIR, DTI, and ASL, which will not be used in this study). The MPRAGE parameters were: TR = 6.6 ms, TE = 3.0 ms, flip angle = 8°, FOV = 256 × 256 mm, matrix size = 256 × 256 mm, number of slices = 165, voxel size = 1 × 1 × 1 mm3. The EPI parameters were: TR = 2000 ms, TE = 20 ms, flip angle= 72°, FOV = 224 × 132 mm, matrix size = 112 × 110 mm, number of slices = 33, voxel size = 2×2×2 mm3 (task scans); TR = 2000 ms, TE = 20 ms, flip angle = 72°, FOV = 224 × 111 mm, matrix size = 112 × 110 mm, number of slices = 37, voxel size = 2×2×2mm3 (resting state scan). Note that the FOV for resting state scans was smaller compared to the task scans, which led some brain regions (i.e. predominantly in the visual cortex and cerebellum) to contain missing values when obtained during rest, but not during task performance. Each scan was carefully reviewed by a neuroradiologist, and any significant findings were reported to the subject’s primary care physician.
2.5. fMRI preprocessing
Images were preprocessed using an in-house developed native space method (Razlighi et al., 2014). Briefly, slice timing correction is performed with FSL slicetimer tool. We used mcflirt (motion correction tools in the FSL package [Jenkinson et al., 2012]) to register all the volumes to a reference image (Jenkinson et al., 2002). The reference image was generated by registering (6 df, 256 bins mutual information, and Sinc interpolation) all volumes to the middle volume and averaging them. We then used the method described in Power et al., 2012 to calculate frame-wise displacement (FD) from the six motion parameters and root mean square difference (RMSD) of the BOLD percentage signal in the consecutive volumes for every subject, and used a threshold of 0.3% (Power et al., 2012). RMSD was computed on the motion-corrected volumes before temporal filtering. The contaminated volumes were detected by the criteria FD > 0.5 mm or RMSD >0.3%. Identified contaminated volumes were replaced with new volumes generated by linear interpolation of adjacent volumes. Volume replacement was done before band-pass filtering (Carp, 2013). The motion-corrected signals were passed through a band-pass filter with the cut-off frequencies of 0.01 and 0.09 Hz. We used flsmaths–bptf to do the filtering in this study (Jenkinson et al., 2012). Finally, we residualized the motion-corrected, scrubbed, and temporally filtered volumes by regressing out the FD, RMSD, left and right hemisphere white matter, and lateral ventricular signals (Birn et al., 2006). Images that had undergone more than 30% scrubbing, were excluded from the dataset.
2.6. Calculation of functional connectivity matrices
T1 image segmentation was done using FreeSurfer v5.1 (Fischl, 2012) and visually checked for any inaccuracy. Corrections were made according to the Freesurfer provided guidelines (https://surfer.nmr.mgh.harvard.edu/fswiki/FsTutorial/TroubleshootingData). The coordinates of the 264 putative functional nodes, derived from a brain-wide graph that can be subdivided into multiple functional systems (e.g. default mode, visual, fronto-parietal (Power et al., 2011)), was transferred to subjects T1 space with non-linear registration of the subjects structural scan to the MNI template using ANTS software package (Avants et al., 2009). A spherical mask with 10 mm radius and centered at each transferred coordinates was generated and intersected with the Freesurfer gray-matter mask to obtain the region of interest (ROI) mask for the 264 functional nodes. An intermodal, intra-subject, rigid-body registration of fMRI reference image and T1 scan was performed with FLIRT with 6 degree of freedom, normalized mutual information as the cost function (Jenkinson and Smith, 2001) and used to transfer all the ROI masks from T1 space to fMRI space. These transferred ROI masks were used to average all the voxels within each mask to obtain a single fMRI time-series for each node. For each subject and each condition (rest, 11 RANN tasks), Pearson correlation coefficients were calculated for all possible pairs among the 264 time-series and Fisher z-transformed. We discarded 4 of these 264 nodes (3 “uncertain” and 1 “default mode” region) for further analysis (and all connectivity pairs associated with them), as more than 20 subjects had missing resting state data in these regions.
2.7. Calculation of task potency maps
Our approach to calculate task potency is similar (although not identical) to earlier papers on this method, of which a detailed explanation is provided elsewhere (Chauvin et al., 2018, 2019). In Matlab R2017a, we created vectors containing all unique pairs among the 260×260 connectivity matrices (i.e. after excluding the 4 nodes described above). We then standardized each task’s vector on a subject level by the group average resting state vector. We decided to standardize by group level resting state data because some individual resting state scans contained missing data for certain ROIs. Specifically, for each subject’s connectivity value in each pair, we subtracted the mean connectivity and divided by the standard deviation for that pair across all participants during resting state. This resulted in 11 “task potency” maps for every individual, reflecting pairwise changes in connectivity from the resting state to each task state. A positive task potency value reflects enhanced synchronicity between nodes during task performance, whereas negative task potency indicates reduced synchronicity (note that this could imply the occurrence of decoupling, but also an increased inverse coupling). Generally, a value that is further away from zero (irrespective of the direction) means a greater change in connectivity from resting state.
2.8. Cortical thickness analysis
Using each individual’s T1-weighted MPRAGE image, cortical thickness measures were derived using the FreeSurfer v5.1 software package (http://surfer.nmr.mgh.harvard.edu/). Although the estimation procedure is automated, we manually checked the accuracy of the spatial registration and the white matter and gray matter segmentations following the analytic procedures outlined by Fjell et al. (2009). Cortical thickness was measured by first reconstructing the gray/white matter boundary and the cortical surface (Dale et al., 1999), and the distances between these surfaces at each point across the cortical mantle were calculated. Using a validated automated labeling system (Fischl et al., 2004), FreeSurfer divided the cortex into 68 different gyral-based parcellations, and calculated the mean thickness in each area. We used the global mean cortical thickness across these 68 areas in our analyses.
3. Statistical analysis
3.1. Demographic and clinical characteristics
We summarized demographic and clinical characteristics of our sample for different age groups, and additionally performed Pearson’s correlation analyses to determine the relationships between age, education, IQ, cognitive performance and global mean cortical thickness.
3.2. Summarizing task potency across tasks within cognitive abilities
Since the RANN tasks were categorized into four latent cognitive abilities on a behavioral level (i.e. vocabulary, episodic memory, processing speed, and fluid reasoning), we examined whether the same clusters would also exist in our fMRI data. If the clusters identified on a neuroimaging level would be in accordance with the four latent cognitive abilities, this would provide face validity for the task potency method in general, and provide a rationale for data reduction by summarizing task potency across tasks belonging to the same cognitive ability. Therefore, we created 11 group average task potency maps, and determined the between-task correlation of potency values across pairs. In addition, we performed a k-means clustering analysis in Matlab (i.e. based on a group-level matrix with rows corresponding to tasks and columns to pairs) to create four clusters, based on the squared Euclidean distance, and a maximum of 100 iterations. We allowed four clustering repetitions with new initial cluster centroid positions, and used the solution with the lowest within-cluster sums of point-to-centroid distances. Based on the outcome (see results section) we created four new individual level potency maps for each cognitive ability, by summarizing task potency values across within-ability tasks.
3.3. Identification of CR-related task-invariant connectivity pairs
To identify task-invariant networks related to CR, we performed linear regression analyses for all four cognitive abilities, with CR factors (i.e. either education or IQ) as predictors and task potency in each pair as the dependent variable. We selected all pairs in which the relationship between task potency and education or IQ was significant (p < .05) across cognitive abilities. Although we did not specify the direction of these relationships, we expected that within-pair relationships would be either consistently positive or negative in each cognitive ability. Furthermore, to account for multiple comparisons, we simulated our analyses with 1000 permutations in a random dataset (i.e. we randomly re-assigned the IQ and education scores among our subjects while maintaining their original task potency maps). With each permutation, we identified the number of pairs that showed significant relationships in the same direction with the CR proxies across cognitive abilities (see Fig. 2). This allowed us to compare the number of task-invariant pairs identified in our “real” dataset with the amount of significant pairs that would be observed by chance alone. To minimize false positive findings, we identified the threshold at which the number of significant pairs was higher than in 95% of the distribution of the permuted data, and only considered education and IQ-related networks in which the number of pairs included were above this threshold for further analysis. To unburden the discussion of the results later on, we already report here that the thresholds for education and IQ were 20 and 19 pairs, respectively. Finally, we also determined which pairs showed a consistent relationship with age, since IQ was collinear with this variable (see Table 2). This step enabled us to determine the degree of overlap between CR- and age-related pairs, as we wanted to evaluate the possibility that the identified task-invariant CR networks actually resulted from an age effect.
Fig. 2. Distribution of the number of pairs that are significant across cognitive abilities in a random dataset.
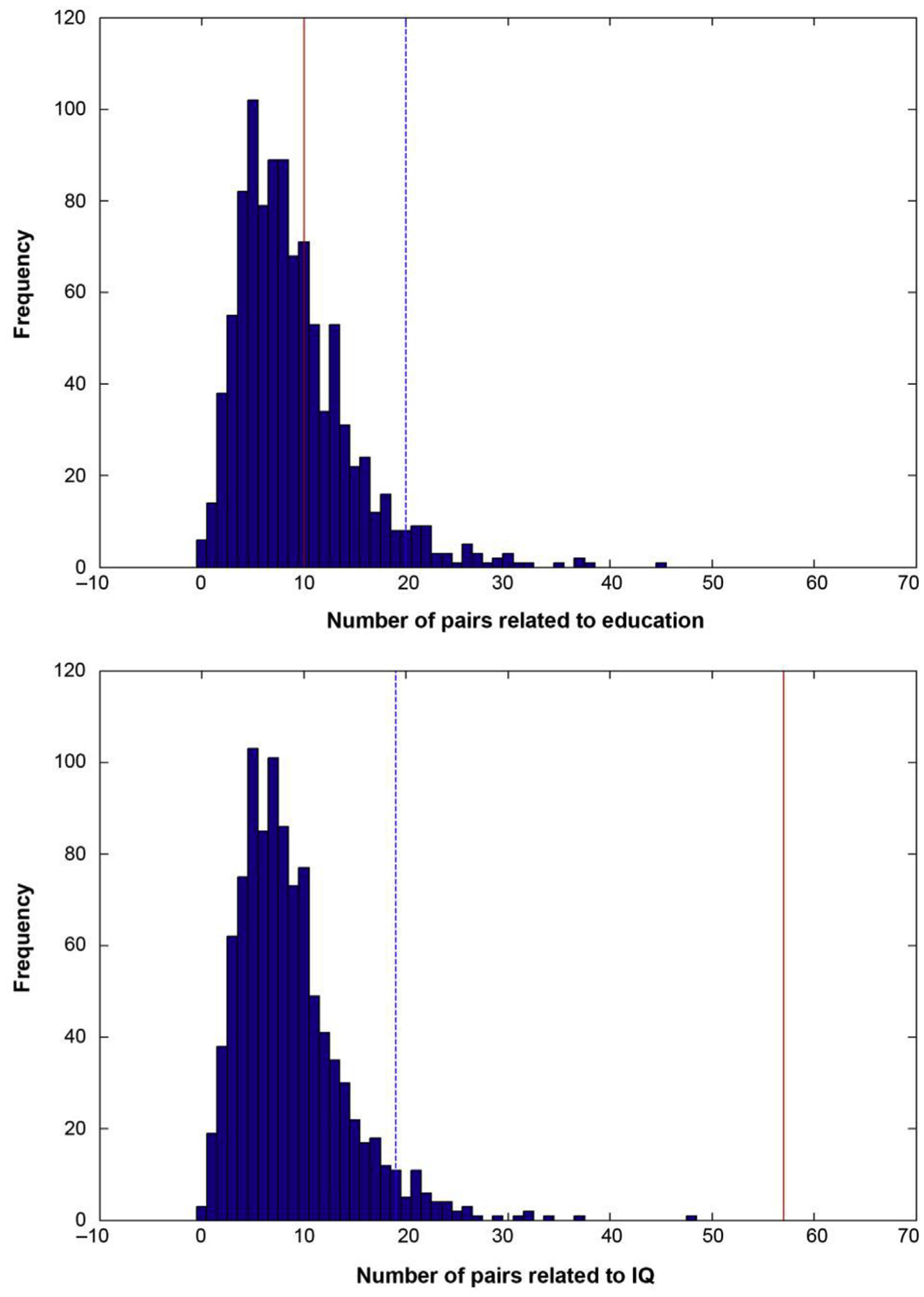
We performed 1000 permutations based on sampling without replacement. Red line = number of pairs found in actual dataset, dotted blue line = threshold at which the number of significant pairs was higher than in 95% of the permuted random dataset.
Table 2.
Relationships between age, CR factors, cortical thickness and cognitive performance.
| Age | IQ | Education | VOCAB | MEM | SPEED | FLUID | Global mean Cth | |
|---|---|---|---|---|---|---|---|---|
| Age | - | .35*** | .11 | .35*** | −.34*** | .54*** | −.32*** | −.52*** |
| IQ | .35*** | - | .50*** | .74*** | .25*** | .09 | .31*** | −.07 |
| Education | .11 | .50*** | - | 37*** | .20** | −.03 | .24*** | −.01 |
| VOCAB | .35*** | .74*** | .37*** | - | .27*** | −.04 | .29*** | −.06 |
| MEM | −.34*** | .25*** | .20** | .27*** | - | −.41*** | .59*** | .24*** |
| SPEED | .54*** | .09 | −.03 | −.04 | −.41*** | - | .30*** | −.24*** |
| FLUID | −.32*** | .31*** | .24*** | .29*** | .59*** | .30*** | - | .29*** |
| Global mean Cth | −.52*** | −.07 | −.01 | −.06 | .24*** | −.24*** | .29*** | - |
Results represent Pearson’s correlation coefficients
= significant at p < .001
= significant at p < .01
= significant at p < .05
IQ = score on the American National Adult Reading Test, Cth = cortical thickness.
3.4. Relationships between task potency and cognitive performance
To test whether the task-invariant CR network(s) identified in the previous step was related to better cognition after taking into account brain structure, we first summarized task potency values across the CR-related pairs to derive one score for each participant. To that end, we performed Principal Component Analysis (PCA) on centered task potency values and then used of each pair’s loadings to the first component to create subject scores. These subjects scores take into account the inter-dependence of task potency values across CR-related pairs, and treat task potency within the task-invariant CR network as one latent construct. A higher task potency summary score indicates that an individual expresses the CR-related task potency pattern to a greater extent. To determine whether higher task potency was associated with better cognition after adjusting for brain structure, we carried out multiple linear regression analyses in which cognitive performance in either episodic memory, processing speed, and fluid reasoning was predicted from global mean cortical thickness (i.e. across 68 parcellations) and the task potency summary score. Vocabulary was not considered in this analysis, as performance within this cognitive ability was unrelated to global mean cortical thickness (i.e. an effect of task potency would not be “above and beyond” the impact of brain structure and thus not truly reflect the CR phenomenon).
Since the CR-related task-invariant network consisted of pairs in which task potency either positively or negatively correlated with education or IQ, we expected the PCA to provide loadings in opposite directions. Therefore, in addition to summarizing task potency across all CR-related pairs, we also subdivided the task-invariant network into pairs with positive and negative loadings (which we multiplied by −1) to the first component. We used these loadings to obtain separate subject scores for each type of pairs, and reran our models with either “positive” or “negative” task potency summary scores as a predictor.
3.5. Sensitivity analyses
As discussed briefly, in addition to the identification of education- and IQ-related task potency pairs, we also determined which pairs were consistently associated with age across cognitive abilities. This was to gain insight into the degree of overlap CR-related pairs and age-related pairs. As the overlap was limited (see results section) we did not exclude any age-related pairs from the task-invariant CR network in our main analyses. However, to reduce the possibility that the identified task-invariant network could be explained by age rather than a CR-related phenomenon, we repeated our analyses described in the previous section after restriction to task potency pairs that only correlated with CR factors and were unrelated to age. In addition, we also performed sensitivity analyses in which task potency pairs with nodes that belonged to the “uncertain” system as labeled by Power et al. (2011) were excluded. We compared our original findings with the results from these sensitivity analyses to evaluate their robustness.
4. Results
4.1. Demographic and clinical characteristics
Table 1 provides demographic features of the study participants. As shown in Table 2, there was a positive correlation between age and IQ (r = 0.35, p < .001). Education and IQ were also positively related (r = 0.50, p < .001). Performance on each cognitive ability was related to scores within the other abilities (except for processing speed and vocabulary). Lower global mean cortical thickness was associated with older age (r = −0.52, p < .001), confirming that this measure captures age-related changes in brain structure. Finally, higher age and lower global mean cortical thickness were related to worse cognitive performance (except for global mean cortical thickness and vocabulary).
Table 1.
Characteristics of our sample according to different age groups.
| Age | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–80 |
|---|---|---|---|---|---|---|
| N | 42 | 47 | 39 | 44 | 49 | 44 |
| Sex (% male) | 31.0 | 31.9 | 56.4 | 54.5 | 55.1 | 45.5 |
| Education (years) | 15.64 ± 2.23 | 16.28 ± 2.39 | 16.13 ± 2.73 | 16.43 ± 1.82 | 16.16 ± 2.30 | 16.80 ± 2.76 |
| IQ | 113.55 ± 8.49 | 112.66 ± 8.94 | 113.72 ± 9.21 | 117.52 ± 7.51 | 119.78 ± 7.58 | 120.65 ± 7.16 |
| DRS total | 140.51 ± 2.51 | 139.89 ± 2.68 | 139.39 ± 2.68 | 140.32 ± 2.99 | 139.67 ± 3.13 | 139.64 ± 2.86 |
| Global mean Cth | 2.70 ± .11 | 2.65 ± .09 | 2.66 ± .09 | 2.61 ± .07 | 2.57 ± .10 | 2.53 ± .11 |
Data are displayed as mean ± SD. IQ = score on the American National Adult Reading Test, DRS = score on the Mattis Dementia Rating Scale, Cth = cortical thickness. Global mean cortical thickness was obtained by averaging across 68 Freesurfer parcellations.
4.2. Summarizing task potency across tasks within cognitive abilities
We used group average task potency maps to determine the between-task correlation of potency values across pair. As shown in Fig. 3, the correlations among within-ability tasks were generally higher than for between-ability tasks. This observation was confirmed by the k-means clustering analysis, which resulted in four clusters that were identical to the RANN latent cognitive abilities (see Supplementary Fig. 1). This provided a rationale for the summarization of task potency values within each cognitive ability, and we thus created four new task potency maps (i.e. for vocabulary, episodic memory, processing speed and fluid reasoning) for each individual by summarizing task potency values across within-ability tasks. These maps were used for further analyses.
Fig. 3. Correlation between group task potency maps for each RANN task.
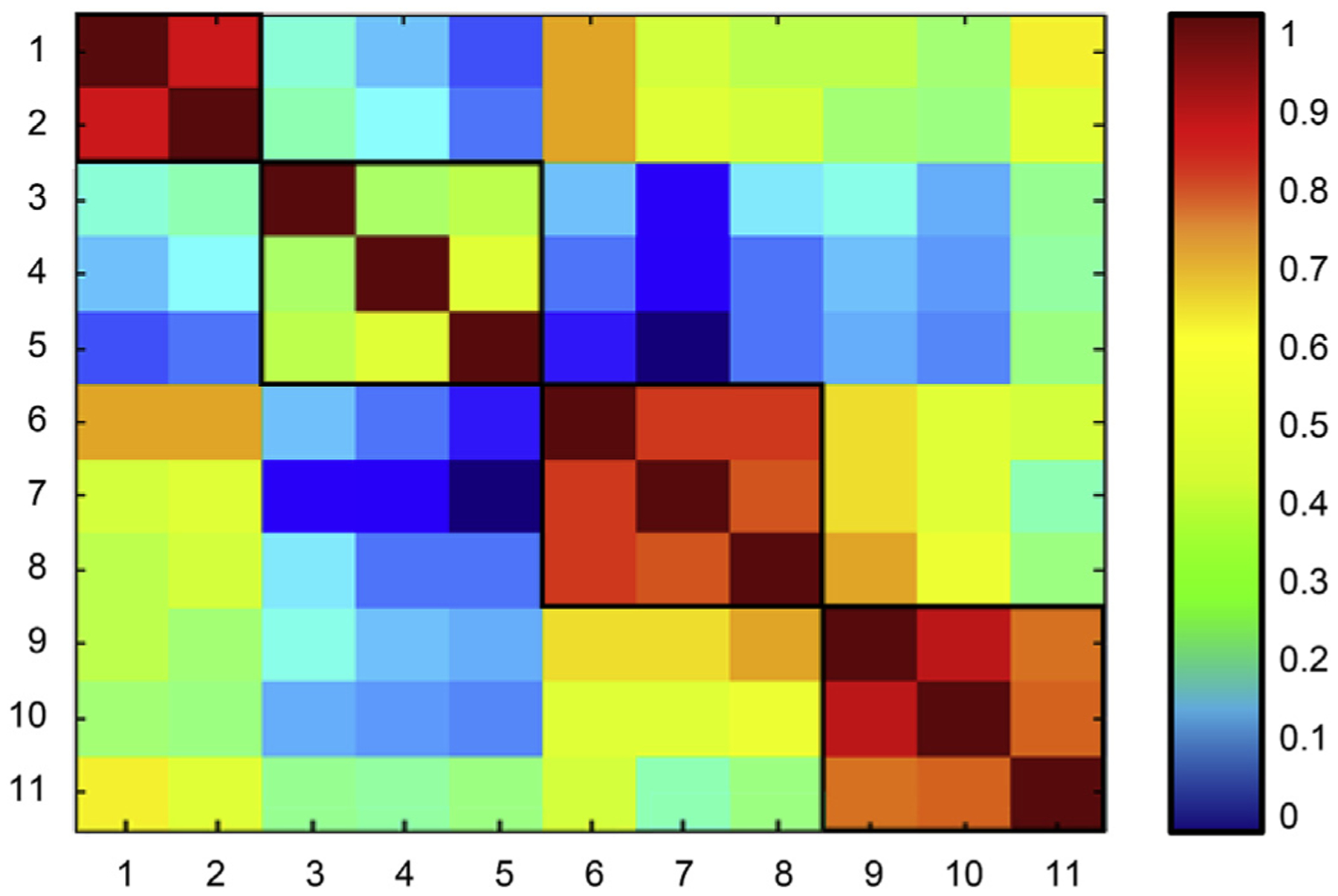
Tasks represented in ascending order: 1. Antonyms, 2. Synonyms (VOCAB); 3. Logical Memory, 4. Paired Associates, 5. Word Order Recognition (MEM); 6. Letter Comparison, 7. Pattern Comparison, 8. Digit Symbol (SPEED); 9. Matrix Reasoning, 10. Paper Folding, 11. Letter Sets (FLUID).
4.3. Identification of CR-related task-invariant connectivity pairs
Linear regression analyses across all four cognitive abilities, revealed 10 pairs in which education was consistently related to task potency. As this number of pairs was below the threshold of 19, as established based on random permutations (see statistical analysis section), the probability that these pairs were observed by chance alone was high and thus we did not consider this network for further analysis. In contrast, we found 57 pairs in which IQ was significantly related to task potency across all cognitive abilities. The ROIs included in these pairs were mainly part of the default mode (21%), fronto-parietal task control (14%) and salience system (9%, see Supplementary Table 2). Among these pairs, there were both positive and negative correlations, but the direction of these relationships across cognitive abilities were always consistent within pairs. 28 pairs were positively related to IQ and 29 pairs showed negative correlations (see Fig. 4a and b, respectively). The negative IQ-related subgroup included all ROI pairs within the default mode system. More generally, the most pronounced systems (i.e. default mode, fronto-parietal, salience) each showed a tendency for negative correlations with IQ. Furthermore, compared to the “positive” IQ-pairs, the “negative” pairs relatively often consisted of between-hemisphere connections (instead of within hemispheres). Finally, there was a high number of pairs (i.e. 1317) in which task potency was related to age, but only 17 of these pairs overlapped with those associated with IQ. This means that the IQ-related task-invariant network was largely unique and could not be explained by an effect of age alone.
Fig. 4. The IQ-related task-invariant network.
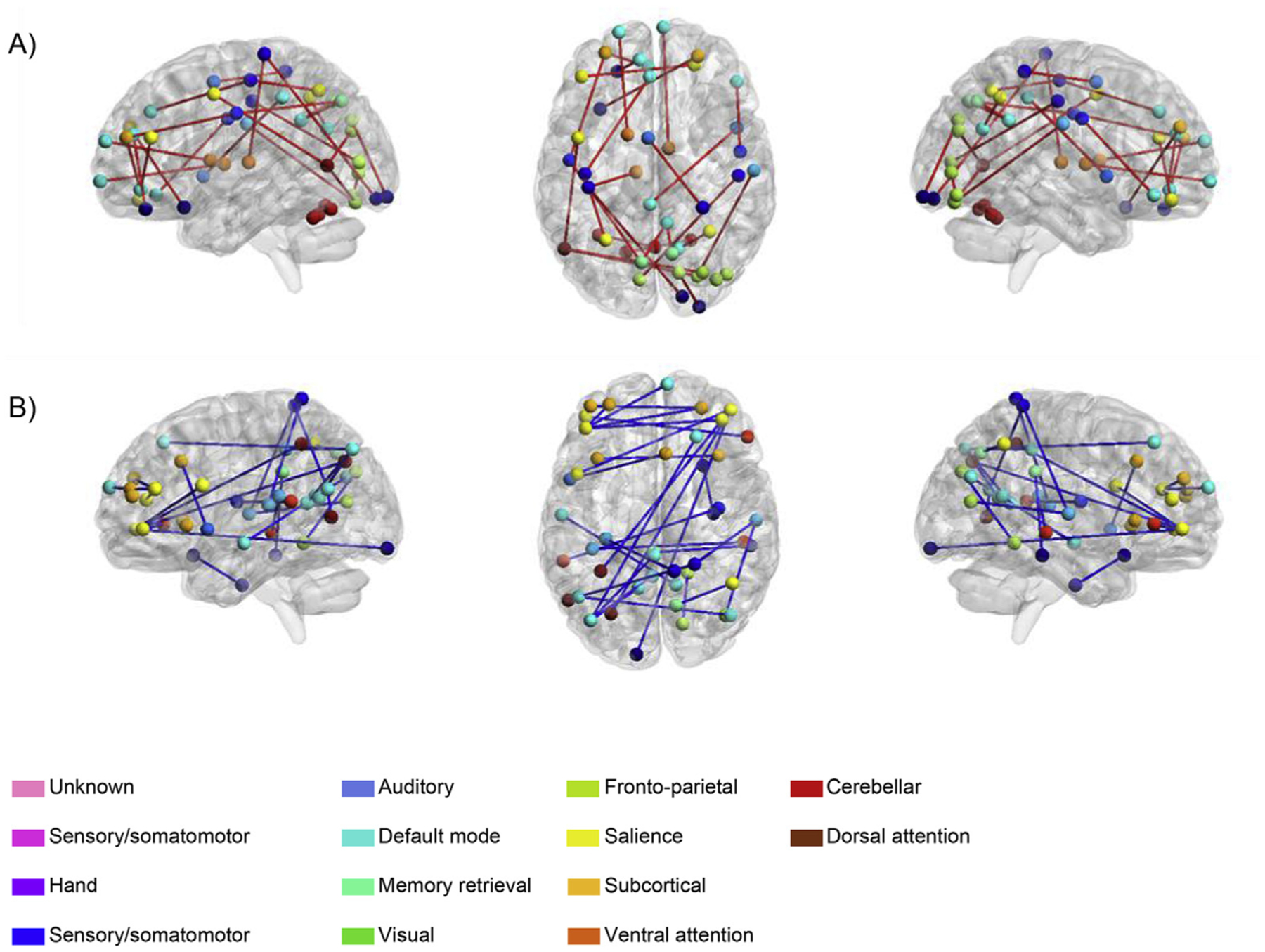
(A) Pairs with positive loadings to the task potency summary score, (B) pairs with negative loadings to the task potency summary score. The task potency summary score was created by performing PCA and using loadings to the first principal component to create subject scores. ROIs with the same color belong to the same system as defined by Power et al. (2011). Edges in blue reflect a negative relationship between IQ and that pair’s task potency, edges in red indicate a positive relationship. This figure was created with the BrainNet Viewer (http://www.nitrc.org/projects/bnv/) (Xia et al., 2013).
4.4. Relationships between task potency and cognitive performance
The results of the PCA to summarize task potency across the 57 IQ-related pairs we identified are listed in Supplementary Table 3. Pairs that positively correlated with IQ consistently showed positive loadings to the first component, whereas all other pairs (which were negatively related to IQ) showed negative loadings. We used the task potency summary score and global mean cortical thickness in multiple regression analyses as predictors of episodic memory, processing speed and fluid reasoning (see Table 3). Global mean cortical thickness was significantly related to performance in all three cognitive abilities (range [absolute] β = 0.22–0.32, p < .001; note that the effect for Speed was inverted as a higher score reflects slower – and thus worse – performance). For both episodic memory and fluid reasoning, we additionally found that greater expression of the IQ-related task potency pattern in the task-invariant network was associated with better performance (β = 0.19, p < .01; β = 0.17, < .01, respectively).There was no relationship between task potency and processing speed (β = 0.06, p = .39).
Table 3.
The effects of global mean cortical thickness and task potency on cognitive performance.
| MEM | SPEED | FLUID | ||||
|---|---|---|---|---|---|---|
| β | P | β | P | β | P | |
| Global mean Cth | .28*** | <.001 | −.22*** | <.001 | .32*** | <.001 |
| TP | .19** | <.01 | .06 | .39 | .17** | <.01 |
β = standardized coefficient, Cth = cortical thickness, TP = task potency in the IQ-related task-invariant network.
= significant at p < .001
= significant at p < .01
= significant at p < .05.
When we repeated these analyses after dividing the task-invariant network into pairs in which task potency showed a positive versus a negative correlation with IQ, we found that while effects of global mean cortical thickness remained similar in all models, the “positive” and “negative” task potency summary scores demonstrated opposite effects on cognition (see Table 4). Specifically, we found that after adjusting for brain structure, greater task potency in pairs that positively related to IQ was associated with better episodic memory (β = 0.18, p < .01) and fluid reasoning (β = 0.13, p < .05), whereas in pairs with negative correlations with IQ, less task potency related to better performance in these cognitive abilities (episodic memory: β = −0.18, p < .01; fluid reasoning: β = −0.20, p < .001).
Table 4.
The effects of global mean cortical thickness and task potency in “positive” versus “negative” pairs on cognitive performance.
| MEM | SPEED | FLUID | ||||
|---|---|---|---|---|---|---|
| β | P | β | P | β | P | |
| Positive | ||||||
| Global mean Cth | .28*** | <.001 | −.22*** | <.001 | .31*** | <.001 |
| TP | .18** | <.01 | .06 | .31 | .13* | .03 |
| Negative | ||||||
| Global mean Cth | .27*** | <.001 | −.23*** | <.001 | .31*** | <.001 |
| TP | −.18** | <.01 | −.04 | .51 | −.20*** | <.001 |
β = standardized coefficient, Cth = cortical thickness, TP = task potency in the IQ-related task-invariant network.
= significant at p < .001
= significant at p < .01
= significant at p < .05.
4.5. Sensitivity analyses
Among the 57 IQ-related task potency pairs in the identified task-invariant network, 17 pairs also showed a consistent correlation with age across cognitive abilities. To reduce possible effects of age, we therefore repeated our analyses with the 40 pairs that exclusively correlated with IQ and were unrelated to age. Furthermore, 7 pairs consisted of nodes that were classified as “uncertain” by Power et al. (2011), and thus we also reran our analyses with the remaining 50 IQ-related pairs only. All results from our sensitivity analyses were virtually the same compared to the original findings (see Supplementary Tables 4 and 5).
5. Discussion
5.1. Summary of results
In this study, we used an adapted version of the task potency method (Chauvin et al., 2018, 2019) to identify a task-invariant CR network in a large group of healthy subjects across the adult age span. This network consisted of 57 pairs of brain regions in which the change in functional connectivity across various cognitive tasks relative to rest (i.e. task potency) was directly related to IQ. 28 task potency pairs were positively correlated to IQ and 29 pairs showed negative correlations. The brain regions involved in this network were predominantly part of the default mode, fronto-parietal task control and salience systems (Power et al., 2011). Suppression of the default mode system is associated with the orientation of attention towards external tasks (Anticevic et al., 2012). The front-parietal task control and salience system have been previously linked to goal-directed behavior and the detection of salient stimuli, respectively (Seeley et al., 2007; Zanto and Gazzaley, 2013). After adjusting for global mean cortical thickness, individuals who expressed the CR-related task potency pattern to a greater extent, performed better on episodic memory and fluid reasoning tasks.
5.2. Previous literature on task-invariant networks
We are not the first to investigate the existence of a task-invariant network underlying CR. In a sample of healthy subjects across the adult age span, Stern et al. (2008) identified a common spatial pattern of task load-related BOLD activity from the encoding phase of a working memory task that required two distinct cognitive processes: verbal (i.e. letter) and object (i.e. shape) encoding. This pattern showed correlations with IQ and vocabulary scores among young individuals, and involved brain regions in the superior and medial frontal gyrus (Stern et al., 2008). Using a more elaborate cognitive task battery, another study in a similar sample performed PCA on block and event-related contrasts of 12 RANN tasks (i.e. the same tasks we currently used, with the addition of Picture Naming) and found that the first principal component constituted a pattern that was common to all tasks, and also correlated with education. Positive loadings to this pattern were found within regions associated with the dorsal attention system, and negative loadings were within areas that were reminiscent of the default mode system (Habeck et al., 2016). In a different study, Stern et al. (2018) used the same RANN data and determined the number of principal components that optimally predicted IQ in a linear regression. Regression weights from this analysis were used to create an IQ-related task-invariant BOLD pattern, and expression of this pattern moderated between cortical thickness and fluid reasoning performance among healthy individuals across the adult age span. The most important areas that contributed to this pattern were the cerebellum, (superior) temporal, (inferior) parietal, precuneus and several regions within the frontal cortex (Stern et al., 2018). A study by Cole et al. (2012) among college students used a working memory task that included a verbal (i.e. words) and non-verbal (i.e. faces) condition. They showed that the lateral prefrontal cortex (LPFC) was involved in both conditions, and conceptualized the involvement of this area as related to cognitive control (i.e. activation levels correlated to the degree of control required, the correctness of the response and overall accuracy). Importantly, they subsequently used resting state fMRI to demonstrate that global connectivity of the LPFC was related to fluid intelligence (Cole et al., 2012). Further applying this finding to CR, Franzmeier and colleagues (2017) showed that resting state global connectivity of the LPFC correlated with education and also acted as a moderator in the relationship between hypometabolism in the precuneus and memory performance among individuals with prodromal Alzheimer’s disease (Franzmeier et al., 2017a; Franzmeier et al., 2017b). Generally, it can be concluded that previous studies on task-invariant CR networks have revealed several overlapping results in terms of brain areas (e.g. frontal, parietal) that appear to be involved, but that it remains difficult to fully reconcile these studies due to differences in fMRI approaches.
In our study, we therefore combined favorable properties of event-related and resting state fMRI techniques described above. That is, using an adapted version of the task potency method introduced by Chauvin and colleagues (2018, 2019), we were able to acquire functional data during task performance, while concurrently taking into account the interconnected nature of the brain. The task potency measure is derived from an integration of both task-related and resting state functional connectivity. Functional connectivity during task performance likely reflects the sum of baseline connectivity and specific changes from this baseline in response to a given task. By extracting the difference between task-related and resting state functional connectivity, we arguably captured highly unique, task-related information. To our knowledge, we are the first to use task potency data to derive a task-invariant network in the context of CR. Interestingly, one study unrelated to CR recently demonstrated the existence of a common network in which task potency related to cognitive performance across three different fMRI tasks (Chauvin et al., 2019). Apart from visual and motor areas (which were expected to be involved due to the visual nature of these tasks and the motor responses that they required), the authors also found “higher--order” temporo-frontal areas to be part of this network, which they attributed to the exertion of cognitive control across all tasks (i.e. a cognitive process that has previously been linked to CR [e.g. Franzmeier et al., 2017a]).
5.3. Relevance of our findings
Our study supports the idea that the neural basis of CR, at least partly, constitutes a generic, task-invariant network. This finding has two important implications. First, such a task-invariant network would be an ideal target for interventions studies. If we succeed at determining how to improve the performance of this network, it could protect individuals with initially low CR against the development of many forms of cognitive impairment (e.g. memory deficits, executive dysfunction) with potentially various etiologies. Treatments aimed at enhancing CR have been suggested as a promising therapy to delay or prevent the emergence of cognitive decline (Stern, 2013). Secondly, the characterization of CR as a single mechanism on a functional brain level provides an alternative operationalization of the concept. Currently, CR is often measured based on proxies, which are easily measurable factors (e.g. education, IQ) that are correlated to the concept (Jones et al., 2011). However, proxies are often relatively static and therefore not suitable to capture within-individual variation in CR that results from the effects of aging and disease (i.e. decreases) or lifestyle enhancements (i.e. increases). Moreover, many proxies are not specific to CR, as they are also correlated with other resilience-related constructs, such as brain maintenance (Reed et al., 2010). Finally, proxies are conceptually unsatisfactory because they fail to distinguish between CR as a hypothetical construct and its determinants. Another increasingly popular approach to quantify CR is the use of residual methods (e.g. van Loenhoud et al., 2017), in which the discrepancy between brain structure and cognitive performance on an individual level is used as a more direct measure of the concept. While useful in a scientific context to elucidate the contributing factors and effects of CR, these methods provide a negative definition of the concept (i.e. explaining a concept by describing what it is not), which is insufficient on a theoretical level. Although replication is needed, quantifying CR based on task potency in the task-invariant network we currently identified, could result in a more concrete, explanatory definition of CR.
5.4. The absence of a task-invariant network for education
We identified a CR network that is task-invariant in the sense that task potency in the respective connectivity pairs correlated with IQ across all four cognitive abilities. In contrast, we did not find a task-invariant network for education. There are multiple explanations for this (absence of a) result. First, it is possible that IQ, as compared to education, is more strongly related to CR (Jefferson et al., 2011; Richards and Sacker, 2003). Indeed, it has been established that more intelligent individuals generally attain higher academic achievement (Deary et al., 2007; Lopes Soares et al., 2015), which suggests that (part of) the effect of education on CR could be explained by IQ. Moreover, education might be a less suitable measure of CR, because it has a more limited variance than IQ, especially in older cohorts and among women (Jones et al., 2011; Starr and Lonie, 2008). It has also been argued that due to differences in ethnic backgrounds or socioeconomic status, academic quality is not uniform across individuals with equal years of education (Manly et al., 2002). Finally, heterogeneity in the type of education is not captured when this variable is measured in years. Individuals who complete many years of education tend to become increasingly specialized in a certain set of cognitive skills. For example, some persons may be particularly well-trained within the language domain, whereas other individuals with the same level of education might have further developed themselves on a mathematical level. As different cognitive abilities are (at least partly) coordinated by distinct, non-overlapping functional networks and activity patterns in the brain (Habeck et al., 2016, 2018; Połczyńska et al., 2017; Stern et al., 2014; Tsukiura et al., 2001), education may be a less suitable candidate for the identification of a task-invariant network of CR. We speculate that education may impart CR through several independent mechanisms that support cognitive performance on a more task-specific level (Chauvin et al., 2019).
5.5. Task potency in the context of RANN
Our data were originally collected in the context of the RANN study, which aims to investigate whether spatial fMRI networks can be derived that are uniquely associated with the performance in each of the four cognitive abilities (i.e. vocabulary, episodic memory, processing speed, and fluid reasoning). Previous results have demonstrated convergent and discriminant validity for the 12 tasks included in the RANN study (i.e. all tasks that were used here, with the addition of Picture Naming) on a behavioral and functional brain level. Specifically, both cognitive performance scores and block-based BOLD activation patterns showed greater similarity between tasks within the same cognitive ability, and reduced similarity between tasks that reflected different cognitive abilities, respectively. In addition, linear indicator regression was used to derive four unique covariance patters for each cognitive ability, which showed good classification accuracy (i.e. identifying the correct task based on an individual’s fMRI activation pattern) in independent samples (Habeck et al., 2016; Stern et al., 2014).
Our results are in line with these reports, as we also found that the four cognitive abilities naturally emerged from our task potency data. That is, comparable to earlier findings, the correlations among group-level task potency maps from within-ability tasks where generally higher than for between-ability tasks, and using a k-means approach, four clusters could be identified that corresponded to each cognitive ability. These results have two important implications. First, it further supports the existence of unique neural networks that underlie four main cognitive abilities that capture most of the age-related variance in cognitive performance. The fact that we replicated earlier findings with a novel fMRI analysis approach, suggests the robustness of these reference ability neural networks. Furthermore, the finding that our neuroimaging data could be summarized in a biologically meaningful manner and thus behaved as expected, provides additional face validity for the task potency method in general and demonstrates its utility and broad applicability.
It is important to note that although our results support the idea that different cognitive abilities have distinct spatial fMRI networks, which meaningfully contributes to the existing body of work on RANN data, this was not the main focus of our current study. In fact, we were specifically interested in a network that was common (instead of unique) for each cognitive ability, to identify a task-invariant network in the context of CR. We were thus able to re-use RANN data for a different purpose compared to the original rationale behind the study.
5.6. Strengths and limitations
This study has several strengths. We selected a relatively large, community-based sample of healthy individuals across a broad age range (i.e. 20–80). Importantly, each of these subjects underwent an elaborate fMRI procedure that included a resting state scan and 11 task-based scans. This within subjects-design is excellent for our research aim to a network that is truly involved in multiple tasks for each individual. Furthermore, we used a novel technique (i.e. task potency) to analyze the fMRI data, which provided unique, task-relevant information about the functional organization of the brain.
One of the limitations of our study is that while most of the cognitive abilities were extensively assessed with three different tasks, our fMRI test battery only included two vocabulary tasks. It is therefore possible that the accuracy with which vocabulary performance was estimated, was somewhat lower compared to the other latent cognitive abilities. On a methodological level, another limitation is the fact that we excluded some subjects from our originally selected sample, as well as removed a set of ROIs from our analyses (i.e. due to large percentages of missing or scrubbed data). Excluded subjects, on average, were older and had a lower cortical thickness than the included sample. This implies that some degree of selection bias was at play, which may have affected the generalizability of our findings. Likewise, the exclusion of ROIs from our analysis may have caused us to overlook potentially relevant connectivity pairs in which task potency is (task-invariantly) related to CR. On a related note, for practical reasons (i.e. missing data for some individuals in certain ROIs during their resting state scans), we calculated task potency values based on group level resting state data, rather than using a subject level approach. There have been recent papers on the task potency technique that describe ways to obtain task potency values entirely based on subject-specific data (Chauvin et al., 2018, 2019). Briefly, this approach entails the subtraction of an individual’s resting state value in each connectivity pair from that subject’s task-based value, instead of standardizing it by the mean and standard deviation of the group average resting state data. This method accounts for possible differences between subjects in functional connectivity during rest, and is thus presumably more tailored towards the individual. On the other hand, without standardization to the group level, comparison between individuals becomes more difficult, which is a potential disadvantage of the subject-level task potency method in comparison to our current approach.
5.7. Future studies
Our findings generate several areas for future study. First, a comparison between different task potency approaches (i.e. based on individual versus group level resting state data) is important to determine which provides the most meaningful data and thus should be the method of choice. Also, longitudinal data are useful to better understand how task potency in the task-invariant network specifically affects trajectories of decline in the four distinct cognitive abilities. Based on this cross-sectional study, we cannot infer whether the CR-related task potency pattern acts through affecting “baseline performance” (e.g. providing an initial advantage that is retained in the face of age- or disease-related structural brain changes) or by influencing longitudinal cognitive changes (e.g. allowing better preservation of cognitive function over time despite structural changes). In addition, since we showed that task potency was correlated with age in many connectivity pairs, it would also be interesting to examine how task potency itself changes over time as individuals age. Furthermore, cross-validation of our results in an independent sample or using different cognitive tests will be important to examine the robustness the task-invariant CR network and its effects on cognitive performance. Finally, other CR factors than education and IQ could be included in our approach. It would be informative to investigate whether measures of occupational attainment, for example, will also result in the identification of a task-invariant CR network, and to what degree it overlaps with the network we found based on IQ.
6. Conclusions
In summary, we demonstrated that CR is (at least partly) related to a functional network that supports cognitive function in a task-invariant manner. The identified task-invariant CR network, in which task potency related to IQ across four latent cognitive abilities, contributes to a better understanding of the mechanisms behind CR. This may in turn facilitate the development of new strategies to enhance CR and thereby minimize the negative impact of age- or disease-related structural brain changes on cognition. In addition, the task-invariant CR network could serve as a useful alternative operational measure of CR in a scientific context.
Supplementary Material
Acknowledgements
We want to thank dr. R.J. Chauvin for her help and support in the implementation of the task potency method in our data. The authors gratefully acknowledge funding support through grant NIH/NIA R01 AG038465 (PI: Stern, Habeck) and NIH/NIA R01AG026158 (PI: Stern). This project has additionally been supported by the foundations “Alzheimer Nederland” and “De Drie Lichten” in The Netherlands. These funding source(s) had no involvement in study design, collection, analysis and interpretation of data, or writing and submission of the report.
Abbreviations:
- CR
cognitive reserve
- RANN
Reference Ability Neural Network study
- NART
American National Adult Reading Test
Footnotes
Declaration of competing interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuroimage.2020.116593.
References
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH, 2012. The role of default network deactivation in cognition and disease. Trends Cognit. Sci 16 (12), 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison N, Song G, 2009. Advanced normalization tools (ANTS). Insight J 2, 1–35. [Google Scholar]
- Arenaza-Urquijo EM, Wirth M, Chételat G, 2015. Cognitive reserve and lifestyle: moving towards preclinical Alzheimer’s disease. Front. Aging Neurosci 10 (7), 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes deLeon CF, Arnold SE, Barnes LL, Bienias JL, 2003. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology 60 (12), 1909–1915. [DOI] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA, 2006. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage 31 (4), 1536–1548. [DOI] [PubMed] [Google Scholar]
- Blessed G, Tomlinson BE, Roth M, 1968. The association between quantitative measures of senile change in the cerebral grey matter of elderly subjects. Br. J. Psychol 114, 797–811. [DOI] [PubMed] [Google Scholar]
- Braun U, Schäfer A, Walter H, Erk S, Romanczuk-Seiferth N, Haddad L, et al. , 2015. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc. Natl. Acad. Sci. U. S. A 112 (37), 11678–11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J, 2013. Optimizing the order of operations for movement scrubbing: comment on Power et al. Neuroimage 76, 436–438. [DOI] [PubMed] [Google Scholar]
- Chauvin RJ, Mennes M, Buitelaar JK, Beckmann CF, 2018. Assessing age-dependent multi-task functional co-activation changes using measures of task-potency. Dev Cogn Neurosci 33, 5–16 pii: S1878–9293(17)30059–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvin RJ, Mennes M, Llera A, Buitelaar JK, Beckmann CF, 2019. Disentangling common from specific processing across tasks using task potency. Neuroimage 184, 632–645. [DOI] [PubMed] [Google Scholar]
- Cole MW, Yarkoni T, Repovs G, Anticevic A, Braver TS, 2012. Global connectivity of prefrontal cortex predicts cognitive control and intelligence. J. Neurosci 32 (26), 8988–8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Strand S, Smith P, Fernandes C, 2007. Intelligence and educational achievement. Intelligence 35 (1), 13–21. [Google Scholar]
- Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9 (2), 179–194. [DOI] [PubMed] [Google Scholar]
- DeSalvo MN, Douw L, Takaya S, Liu H, Stufflebeam SM, 2014. Task-dependent reorganization of functional connectivity networks during visual semantic decision making. Brain Behav 4 (6), 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman HH, Dermen D, 1976. Manual for Kit of Factor-Referenced Cognitive Tests (Princeton) [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, et al. , 2004. Automatically parcellating the human cerebral cortex. Cerebr. Cortex 14 (1), 11–22. [DOI] [PubMed] [Google Scholar]
- Fischl B, 2012. FreeSurfer. NeuroImage. 62 (2), 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Walhovd KB, 2009. High consistency of regional cortical thinning in aging across multiple samples. Cerebr. Cortex 19 (9), 2001–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME, 2006. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat. Neurosci 9 (1), 23–25. [DOI] [PubMed] [Google Scholar]
- Franzmeier N, Caballero MÁA, Taylor ANW, Simon-Vermot L, Buerger K, Ertl-Wagner B, et al. , 2017a. Alzheimer’s Disease Neuroimaging Initiative, 2017a. Resting-state global functional connectivity as a biomarker of cognitive reserve in mild cognitive impairment. Brain Imaging Behav 11 (2), 368–382. [DOI] [PubMed] [Google Scholar]
- Franzmeier N, Duering M, Weiner M, Dichgans M, Ewers M, 2017b. Alzheimer’s Disease Neuroimaging Initiative, 2017b. Left frontal cortex connectivity underlies cognitive reserve in prodromal Alzheimer disease. Neurology 88 (11), 1054–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Castillo J, Bandettini PA, 2017. Task-based dynamic functional connectivity: recent findings and open questions. NeuroImage S1053–8119 (17), 30653–30655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Sliwinski M, 1991. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J. Clin. Exp. Neuropsychol 13, 933–949. [DOI] [PubMed] [Google Scholar]
- Groot C, Hooghiemstra AM, Raijmakers PGHM, van Berckel BNM, Scheltens P, Scherder EJA, van der Flier WM, Ossenkoppele R, 2016. The effect of physical activity on cognitive function in patients with dementia: a meta-analysis of randomized control trials. Ageing Res. Rev 25, 13–23. [DOI] [PubMed] [Google Scholar]
- Habeck C, Gazes Y, Razlighi Q, Steffener J, Brickman A, Barulli D, Salthouse T, Stern Y, 2016. The Reference Ability Neural Network Study: life-time stability of reference-ability neural networks derived from task maps of young adults. Neuroimage 125, 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck C, Eich T, Razlighi R, Gazes Y, Stern Y, 2018. Reference ability neural networks and behavioral performance across the adult life span. Neuroimage 172, 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, et al. , 2013. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 80, 360–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE, 2010. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol 20 (8), 519–534. [DOI] [PubMed] [Google Scholar]
- Jefferson AL, Gibbons LE, Rentz DM, Carvalho JO, Manly J, Bennett DA, Jones RN, 2011. Life course model of cognitive activities, socioeconomic status, education, reading ability, and cognition. J. Am. Geriatr. Soc 59 (8), 1403–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM, 2012. FSL. NeuroImage. 62 (2), 782–790. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S, 2001. A global optimisation method for robust affine registration of brain images. Med. Image Anal 5 (2), 143–156. [DOI] [PubMed] [Google Scholar]
- Jones RN, Manly J, Glymour MM, Rentz DM, Jefferson AL, Stern Y, 2011. Conceptual and measurement challenges in research on cognitive reserve. J. Int. Neuropsychol. Soc 17, 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loenhoud AC, Wink AM, Groot C, Verfaillie SCJ, Twisk J, Barkhof F, et al. , 2017. A neuroimaging approach to capture cognitive reserve: application to Alzheimer’s disease. Hum. Brain Mapp 38 (9), 4703–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes Soares D, Lemosa GC, Primi R, Almeida LS, 2015. The relationship between intelligence and academic achievement throughout middle school: the role of students’ prior academic performance. Learn. Indiv Differ 41, 73–78. [Google Scholar]
- Manly JJ, Jacobs DM, Touradji P, Small SA, Stern Y, 2002. Reading level attenuates differences in neuropsychological test performance between African American and White elders. J. Int. Neuropsychol. Soc 8 (3), 341–348. [DOI] [PubMed] [Google Scholar]
- Mattis S, 1988. Dementia Rating Scale (DRS). Psychological Assessment Resources, Odessa, FL. [Google Scholar]
- Mennes M, Kelly C, Colcombe S, Castellanos FX, Milham MP, 2013. The extrinsic and intrinsic functional architectures of the human brain are not equivalent. Cerebr. Cortex 23 (1), 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Połczyńska M, Japardi K, Curtiss S, Moody T, Benjamin C, Cho A, et al. , 2017. Improving language mapping in clinical fMRI through assessment of grammar. Neuroimage: Clinical. 15, 415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59 (3), 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. , 2011. Functional network organization of the human brain. Neuron 72 (4), 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JC, 1962. Advanced Progressive Matrices, Set II. H.K. Lewis, London, UK. [Google Scholar]
- Razlighi QR, Habeck C, Steffener J, Gazes Y, Zahodne LB, Mackay-Brandt A, Stern Y, 2014. Unilateral disruptions in the default network with aging in native space. Brain Behav 4 (2), 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BR, Mungas D, Farias ST, Harvey D, Beckett L, Widaman K, Hinton L, DeCarli C, 2010. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain 133 (8), 2196–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz DM, Huh TJ, Sardinha LM, Moran EK, Becker JA, Daffner KR, Sperling RA, Johnson KA, 2007. Intelligence quotient-adjusted memory impairment is associated with abnormal single photon emission computed tomography perfusion. J. Int. Neuropsychol. Soc 13, 821–831. [DOI] [PubMed] [Google Scholar]
- Richards M, Sacker A, 2003. Lifetime antecedents of cognitive reserve. J. Clin. Exp. Neuropsychol 25, 614–624. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL, 1991. Decomposing adult age differences in working memory. Dev. Psychol 27, 763–776. [Google Scholar]
- Salthouse TA, 1993. Speed and knowledge as determinants of adult age differences in verbal tasks. J. Gerontol 48, 29–36. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, 2005. Relations between cognitive abilities and measures of executive functioning. Neuropsychology 19, 532–545. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Pink JE, Tucker-Drob EM, 2008. Contextual analysis of fluid intelligence. Intelligence 36, 464–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, 2009. Decomposing age correlations on neuropsychological and cognitive variables. J. Int. Neuropsychol. Soc 15, 650–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, Stern Y, 2009. Physical activity, diet, and risk of Alzheimer disease. J. Am. Med. Assoc 302, 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Zarahn E, Anderson KE, Habeck CG, Hilton J, Flynn J, et al. , 2003. Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Arch. Neurol 60, 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. , 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci 27 (9), 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. , 2009. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A 106 (31), 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr J, Lonie J, 2008. Estimated pre-morbid IQ effects on cognitive and functional outcomes in Alzheimer disease: a longitudinal study in a treated cohort, 8 (1), 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Zarahn E, Habeck C, Holtzer R, Rakitin BC, Kumar A, et al. , 2008. A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cerebr. Cortex 18 (4), 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, 2012. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 11 (11), 1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, 2013. Cognitive reserve: implications for assessment and intervention. Folia Phoniatrica Logop. 65 (2), 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Habeck C, Steffener J, Barulli D, Gazes Y, Razlighi Q, Shaked D, Salthouse T, 2014. The reference ability neural network study: motivation, design, and initial feasibility analyses. Neuroimage 103, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Habeck C, 2018. Deriving and testing the validity of cognitive reserve candidates In: Perneczky RG (Ed.), Biomarkers for Preclinical Alzheimer’s Disease, pp. 63–70. [Google Scholar]
- Stern Y, Gazes Y, Razlighi Q, Steffener J, Habeck C, 2018. A task-invariant cognitive reserve network. Neuroimage 78, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavor I, Parker Jones O, Mars RB, Smith SM, Behrens TE, Jbabdi S, 2016. Task-free MRI predicts individual differences in brain activity during task performance. Science 352 (6282), 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiura T, Fujii T, Takahashi T, Xiao R, Inase M, Iijima T, Yamadori A, Okuda J, 2001. Neuroanatomical discrimination between manipulating and maintaining processes involved in verbal working memory; a functional MRI study. Brain Res Cogn Brain Res 11 (1), 13–21. [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev P, 2006. Brain reserve and cognitive decline: a non-parametric systematic review. Psychol. Med 36, 1065–1073. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Aggarwal NT, Boyle PA, Hebert LE, Mendes de Leon CF, Evans DA, 2010. Cognitive activity and the cognitive morbidity of Alzheimer disease. Neurology 75, 990–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA, 2013. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology 81, 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y, 2013. BrainNet Viewer: a network visualization tool for human brain connectomics. PloS One 8 (7), e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A, 2013. Fronto-parietal network: flexible hub of cognitive control. Trends Cognit. Sci 17 (12), 602–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


