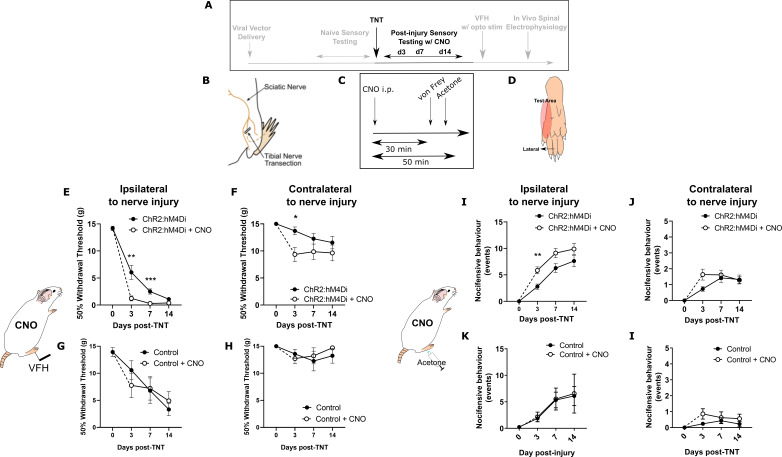
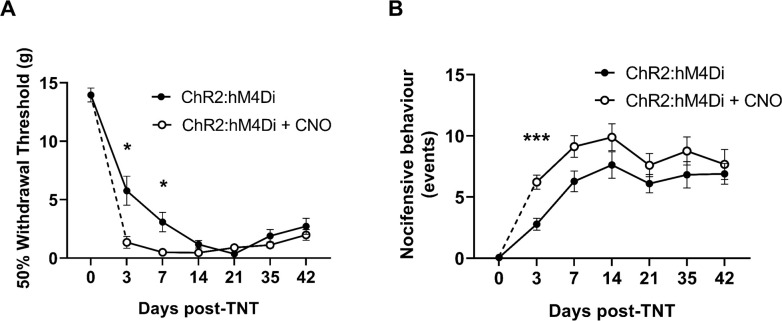
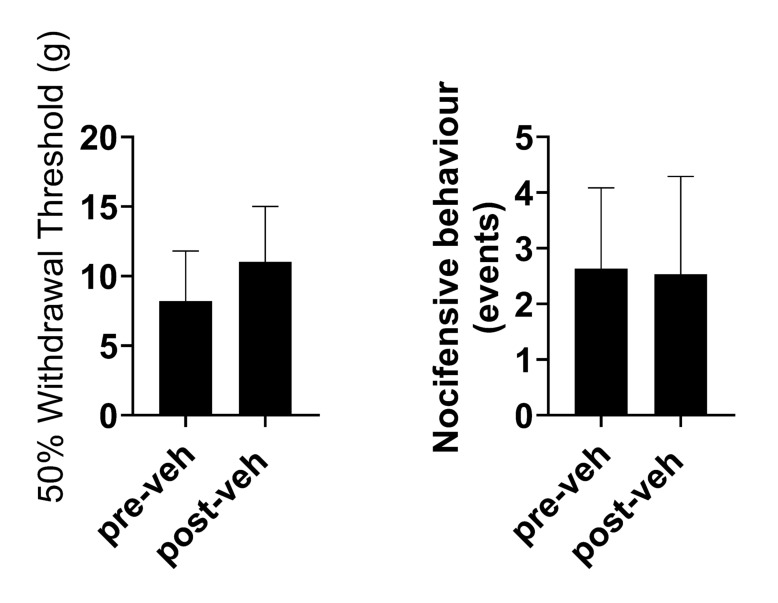
Figure 3. Inhibition of PrL-P neurons unmasks hypersensitivity in neuropathic rats.
(A) Experimental timeline. (B) Tibial nerve transection (TNT) was used to produce the neuropathic injury. (C)- Sensory testing was conducted at 30 min after systemic delivery of CNO and (D) testing was conducted on the lateral plantar surface of the hindpaw in a receptive field adjacent to injured tibial nerve. (E) In TNTPrL.ChR2:hM4Di rats, CNO (2.5 mg·kg−1 i.p.) reduced the mechanical withdrawal threshold at 3 and 7 days post nerve injury on the ipsilateral (injured) hindpaw (two-way ANOVA, main effect CNO, F(1,30)=20.09, p=0.0001; timexCNO, F(2, 60)=6.892, p=0.002; Sidak’s post-test day 3, p=0.008; day 7, p=0.001, n = 16) and (F) on the contralateral paw at 3 days post-injury (two-way ANOVA, CNO F(1,30)=5.77, p=0.02; Sidak’s post-test, p=0.02, n = 16). (G and H) In TNTPrL.Control rats, the same dose of CNO did not alter mechanical withdrawal thresholds on either the ipsilateral or contralateral hindpaw (two-way ANOVA, main effect; ipsilateral CNO, F(1,14)=0.02, p=0.90, n=8 and contralateral CNO, F(1,14)=0.15, p=0.71, n=8, respectively). (I and J) In TNTPrL.ChR2:hM4Di rats, CNO increased acetone-evoked nocifensive events at 3 days post-injury on the ipsilateral paw (two-way ANOVA, main effect CNO, F(1,30)=9.6, p=0.004; Sidak’s post-test, p=0.003, n=16) but not contralaterally (two-way ANOVA, main effect CNO, F(1,29)=1.3, p=0.26, n=16). (K and I) In TNTPrL.Control rats, CNO did not alter acetone-evoked nocicfensive behaviour (two-way ANOVA, main effect CNO ipsilateral, F(1,12)=0.02, p=0.89, n=7 and main effect CNO contralateral, F(1,12)=2.2, p=0.16, n=7).