Graphical abstract
Keywords: Antiviral research, Drug discovery, Tenofovir, Brivudine, HIV, HBV, HSV
Abstract
Since the 1950s, great efforts have been made to develop antiviral agents against many infectious diseases such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), human cytomegalovirus (HCMV), herpes simplex virus (HSV), and varicella-zoster virus (VZV). Among the list of nearly 106 antiviral agents approved in the past five decades, Prof. Erik De Clercq has contributed to the development of 7 antiviral drugs: tenofovir disoproxil fumarate (Viread®) for HIV and HBV treatment, tenofovir alafenamide (Vemlidy®) for HIV and HBV treatment, brivudine (Zostex®) for HSV-1 and VZV treatment, valacyclovir (Valtrex®) for HSV and VZV treatment, adefovir dipivoxil (Hepsera®) for HBV treatment, stavudine (Zerit®) for HIV treatment, and cidofovir (Vistide®) for treating HCMV retinitis in AIDS patients. In addition to the above antiviral drugs, his contributions include two anti-cancer drugs: rabacfosadine (Tanovea®-CA1) for canine lymphoma and plerixafor (Mozobil®) for multiple myeloma and non-Hodgkin’s lymphoma. These achievements are driven by his life-long passions for antiviral research and successful collaborations worldwide. To honor the 80th birthday of Prof. Erik De Clercq, this study highlights his scientific achievements and the importance of life-long passions and collaborations in the success of antiviral research and drug development.
1. Introduction
In the past five decades, many compounds have been approved by the U.S. Food and Drug Administration (FDA) and/or the European Medicines Agency to treat infectious diseases caused by contagious viruses such as HIV, HBV, HCV, HCMV, HSV, VZV, respiratory syncytial virus (RSV), influenza virus, orthopoxviruses, Ebola virus, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In our previous review, we summarized 90 antiviral drugs approved during the period from 1959 to 2016 [1]. This drug list could be extended with at least 16 antiviral agents, which were approved by the U.S. FDA from 2016 to 2020, including (i) four HIV inhibitors: bictegravir, doravirine, fostemsavir, and ibalizumab-uiyk; (ii) four HCV inhibitors: velpatasvir, voxilaprevir, glecaprevir, and pibrentasvir; (iii) four ebolavirus monoclonal antibodies: ansuvimab-zykl, atoltivimab, maftivimab, and odesivimab-ebgn; (iv) one HCMV inhibitor: letermovir; (v) one orthopoxvirus inhibitor: tecovirimat; (vi) one influenza inhibitor: baloxavir marboxil; and (vii) one SARS-CoV-2 inhibitor: remdesivir. Among 106 approved antiviral agents, Prof. Erik De Clercq has contributed to the development of 7 antiviral drugs (Table 1 ). Of note, tenofovir disoproxil fumarate and tenofovir alafenamide are two important antiviral drugs in the treatment of HIV and HBV infections worldwide. His contributions also include plerixafor and rabacfosadine - two antiviral compounds that were repurposed for lymphoma treatment [2], [3]. Due to his lifetime achievements in antiviral treatment (e.g., AIDS drug cocktails), he received the European Inventor Award in 2008 and shared Dr. Paul Janssen Award for Biomedical Research with Dr. Anthony S. Fauci in 2010.
Table 1.
A summary of 9 approved compounds co-contributed by Prof. Erik De Clercq.
| Drug name* | Brand name | Diseases | Approval date | Current status | Ref.* |
|---|---|---|---|---|---|
| Stavudine (d4T) | Zerit® | HIV | 1994/02/08 | Discontinued due to off-target toxicity and drug resistance. | [17] |
| Valacyclovir (US) or Valaciclovir (UK) |
Valtrex®, Zelitrex® |
Cold sores, genital herpes, herpes zoster, chickenpox | 1995/06/23 | Valacyclovir is an important drug for HSV and VZV treatment. | [14] |
| Cidofovir (GS-0504) | Vistide® | HCMV retinitis in AIDS patients | 1996/06/26 | Discontinued due to lack of AIDS patients infected with HCMV. | [35] |
| Brivudine (RP-101) | Zostex® and others | VZV, HSV-1 | 2000/01 | Brivudine in once-daily tablets is an important drug to treat HSV-1 and VZV. | [9], [10] |
| Tenofovir disoproxil fumarate (TDF, GS-4331) | Viread® | HIV, HBV | 2001/10/26 | TDF is an important drug for HIV and HBV treatment. | [23] |
| Adefovir dipivoxil (GS-0840) | Hepsera® | HBV | 2002/09/20 | Replaced by better drugs (e.g., TDF, TAF) | [16] |
| Plerixafor (AMD3100) | Mozobil® | Multiple myeloma, non-Hodgkin’s lymphoma | 2008/12/15 | Plerixafor is mostly reserved for patients who fail stem cell mobilization by conventional therapies [2]. | [28], [29] |
| Tenofovir alafenamide (TAF, GS-7340) | Vemlidy® | HIV, HBV | 2016/11/10 | TAF has superseded TDF to treat HIV and HBV. | [23] |
| Rabacfosadine (GS-9219) | Tanovea®-CA1 | Canine lymphoma | 2016/12/09 | The first FDA conditionally approved drug for canine lymphoma treatment. | [35] |
*: Only key references (co–)authored by Prof. Erik De Clercq were listed.
Prof. Dr. Erik De Clercq was born in Dendermonde, Belgium on 28 March 1941 and received his higher education of bachelor, master, M.D., and Ph.D. at the KU Leuven in Belgium. After his two-year research training at the Stanford Medical Center (1968 to 1970), he returned to Belgium and established his entire academic career at the Rega Institute of KU Leuven. At the age of 34 years, he became one of the youngest full professors in the history of KU Leuven. Since 2006, he has been serving as an emeritus professor and has voluntarily continued his academic research and teaching with a great passion. Due to his scientific contributions and worldwide collaborations, he received Honorary Doctorates from eight prestigious universities: Ghent University (Belgium) in 1997, University of Athens (Greece) in 1997, University of Ferrara (Italy) in 2000, Shandong University (China) in 2005, Charles University (Czech Republic) in 2007, University of South Bohemia (Czech Republic) in 2009, University of Tours (France) in 2010, and University of Hull (United Kingdom) in 2011. On 8 June 2013, he was also recognized as the Honorary Citizen of Hamme – the hometown of Prof. Erik De Clercq. Although it was unfortunate for him to retire at the age of 65 years due to the official requirement in Belgium, there is no rule to stop a free mind to explore the curiosity of science and the eternal goal of curing infectious diseases in all human beings.
Since the beginning of his career in 1957, Prof. Erik De Clercq has contributed with more than 2800 scientific publications and 100 patents, mostly in the field of antiviral research. Furthermore, he has built profound collaborations worldwide and their joint efforts have led to many important discoveries, briefly summarized below.
-
•
In 1968, the discovery of several inducers of interferons (i.e., polyacrylic acid) [4], [5].
-
•
In 1979, the first report of suramin as a potent inhibitor of the reverse transcriptase of animal retroviruses in cell cultures [6]. Suramin was later recognized as the first compound ever to be confirmed as a reverse transcriptase inhibitor of HIV infections in cell cultures [7] and humans [8].
-
•
In 1979, the discovery of brivudine as a potent and selective compound against HSV-1 [9] and VZV [10]. Brivudine was later marketed for the treatment of herpes zoster.
-
•
In 1978, the discovery of (S)-9-(2,3-Dihydroxypropyl)adenine as the first acyclic nucleoside with antiviral activities [11].
-
•
In 1980, the cloning and expression of human β-interferon as well as the elucidation of its primary structure [12], [13].
-
•
In 1982, the identification of amino acid esters, which led to the valine ester of acyclovir so-called valacyclovir with enhanced bioavailability [14], [15].
-
•
In 1986, the discovery of acyclic nucleoside phosphonates as a new class of broad-spectrum anti-DNA virus inhibitors [16].
-
•
In 1987, the discovery of 2́,3́-dideoxy-2́,3́-didehydrothymidine [17], later marketed as stavudine (d4T) for HIV treatment.
-
•
In 1989 and 1990, the discovery of HEPT [18], [19] and TIBO [20] derivatives that were subsequently recognized as the prototypes of non-nucleoside reverse transcriptase inhibitors (NNRTIs) such as rilpivirine [21], [22].
-
•
In 1993, the discovery of [(R)-9-(2-phosphonylmethoxypropyl)adenine] [23], which was later developed as tenofovir for HIV and HBV treatment.
-
•
In 1998, the synthesis of the tenofovir prodrug called tenofovir disoproxil fumarate (TDF) [24]. In 2005 a new prodrug of tenofovir called tenofovir alafenamide (TAF) was developed with improved safety profiles. Both TDF and TAF have been marketed for the treatment of HIV and HBV. As of January 2021, TDF or TAF plus emtricitabine are the only approved regimens for HIV prevention. More than 10 regimens of highly active antiretroviral therapy (HAART) containing either TDF or TAF have been marketed worldwide.
-
•
In 2000, the discovery of a new nucleoside analogue called Cf-1743 [25], [26]. This compound displayed better potency than brivudine [27] and offered great potential in the form of its valine ester (fermavir, FV-100) to treat VZV.
-
•
In 2008, the approval of plerixafor (AMD3100) - a bicyclam derivative originally described as an anti-HIV agent in 1992 [28] (a publication sponsored by the Nobel Prize winner Max Perutz) and 1994 [29]. Plerixafor was licensed as a stem cell mobilizer (Mozobil®) for the autologous transplantation in patients with multiple myeloma or non-Hodgkin’s lymphoma [30], [31], [32], [33], [34].
-
•
In 2016, the approval of rabacfosadine (GS-9219) as a treatment of lymphoma in dogs (Tanovea®-CA1) [3]. Rabacfosadine is a new phosphonate analogue derivatized from PMEG [9-(2-phosphonylmethoxyethyl)guanine] and its antiviral activity was first described by Erik De Clercq et al. in 1987 [35].
To celebrate the 80th birthday of Prof. Erik De Clercq on 28 March 2021, we, as his students, prepared this article to highlight his lifetime achievements as well as important lessons that we have learned from our revered professor. The clinical efficacy and safety of approved drugs, to which Prof. Erik De Clercq contributed, were summarized based on randomized clinical trials. Anecdotes and stories were shared by Prof. Erik De Clercq and confirmed by personal communications.
2. Clinical significance of 6 approved drugs
In the past five decades, Prof. Erik De Clercq and his collaborative teams have contributed to the development of 9 approved compounds, including (i) tenofovir disoproxil fumarate (Viread®) and tenofovir alafenamide (Vemlidy®) for the treatment of HIV and HBV (Fig. 1 a); (ii) valacyclovir (Zelitrex®, Valtrex®) for the treatment of HSV and VZV (Fig. 2 a); (iii) brivudine (Zostex®, Zostavir®, Zerpex®, and other brand names) for the treatment of HSV-1 and VZV (Fig. 2b); (iv) plerixafor (Mozobil®) for the treatment of multiple myeloma and non-Hodgkin’s lymphoma in humans (Fig. 3 ); (v) rabacfosadine (Tanovea®-CA1) for the treatment of canine lymphoma (Fig. 4 ); (vi) cidofovir (Vistide®) for the treatment of HCMV retinitis in AIDS patients; (vii) adefovir dipivoxil (Hepsera®) for HBV treatment; and (viii) stavudine (Zerit®) for HIV treatment. The former six compounds are actively applied in clinical practice, while the latter three compounds are currently discontinued (Table 1).
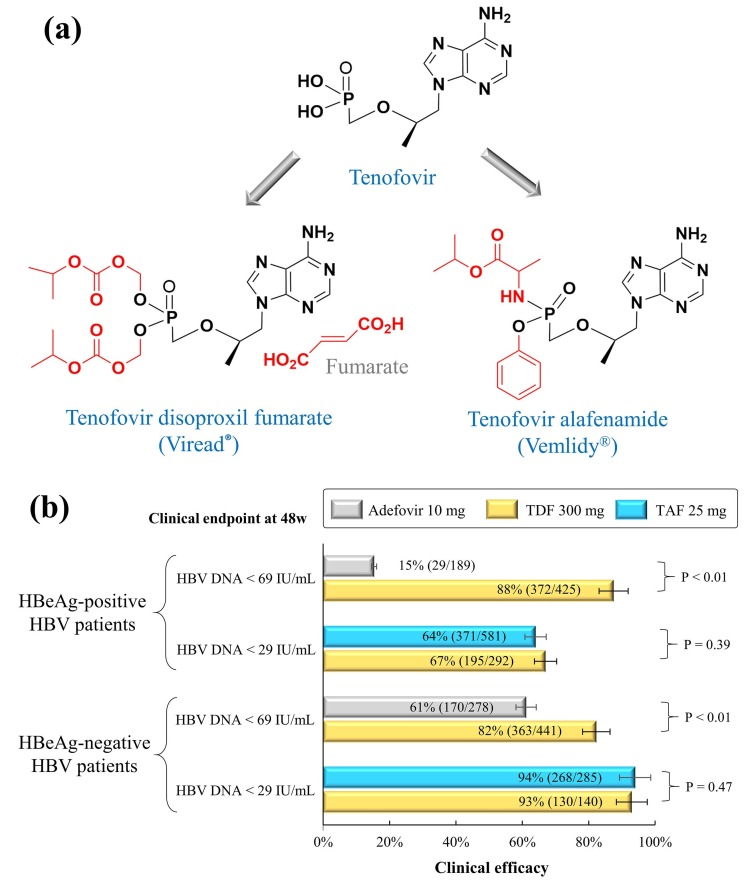
Fig. 1.
Structure and efficacy of TDF and TAF monotherapy in the HBV treatment. (a) TDF and TAF are the prodrugs of tenofovir. (b) Clinical efficacy of TDF and TDF in HBV-infected patients treated with either adefovir dipivoxil 10 mg or TDF 300 mg, TAF 25 mg. Efficacy data from phase 3 or 4 trials were summarized in Table 2.
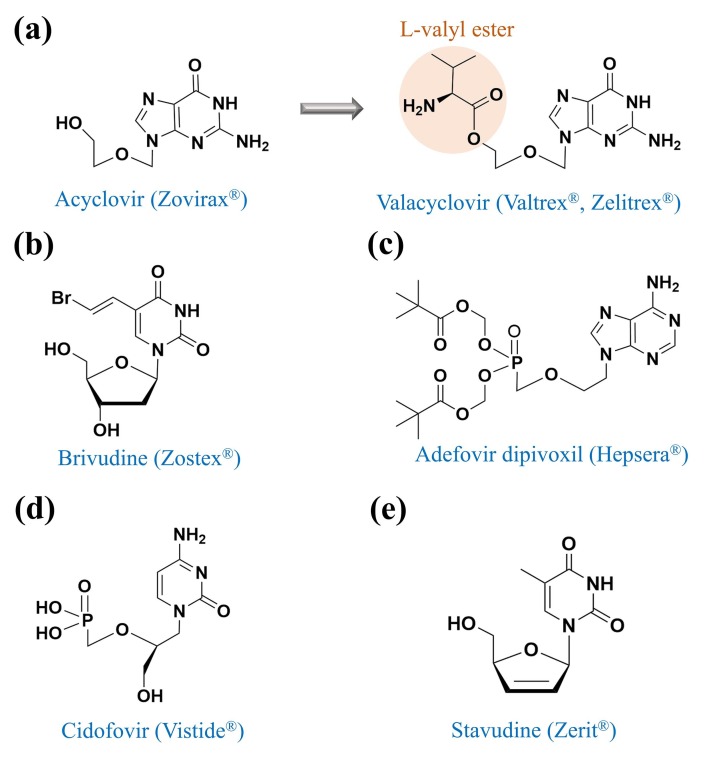
Fig. 2.
Chemical structures of acyclovir, valacyclovir, brivudine, adefovir dipivoxil, cidofovir, and stavudine.
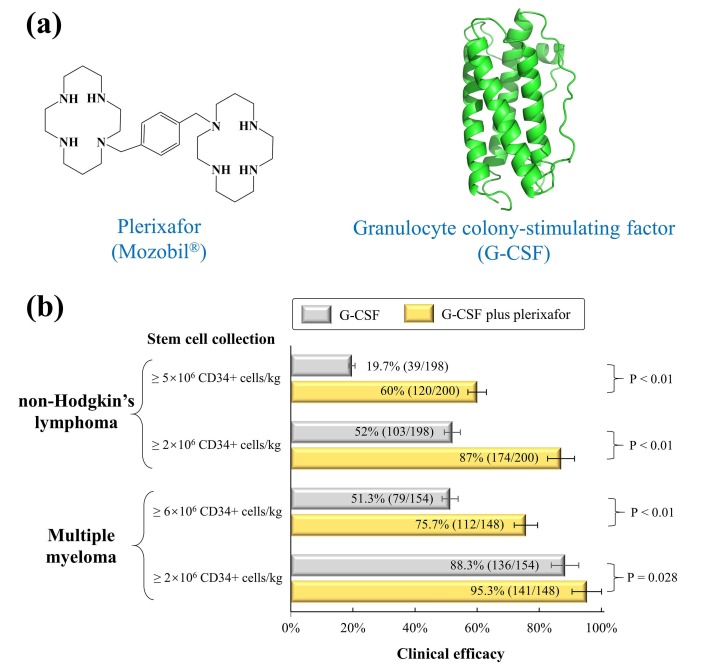
Fig. 3.
Structures and efficacy of plerixafor plus G-CSF. (a) Structures of plerixafor and G-CSF (PDB: 2D9Q). (b) Clinical efficacy of plerixafor and G-CSF in the treatment of non-Hodgkin’s lymphoma and multiple myeloma. Efficacy data from phase 3 studies were summarized in Table 4.
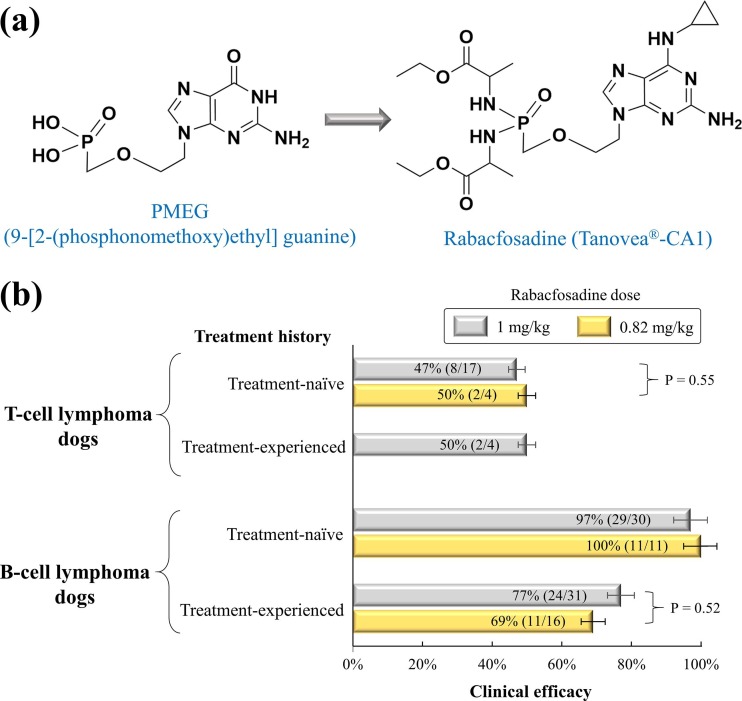
Fig. 4.
Development and clinical efficacy of rabacfosadine in clinical trials. (a) Development of rabacfosadine from the PMEG. (b) Clinical efficacy of rabacfosadine in the treatment of canine lymphoma. Efficacy data were summarized in Table 5.
Since the drug discovery and molecular mechanisms of the above compounds were reviewed by previous studies [1], [2], [36], [37], [38], this section focuses on the clinical applications of six active drugs (tenofovir disoproxil fumarate, tenofovir alafenamide, brivudine, valacyclovir, plerixafor, rabacfosadine).
2.1. Tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF)
Tenofovir is an adenosine nucleotide analogue that actively inhibits HIV-1 and HIV-2 – an important discovery that was first reported by the research team of Prof. Erik De Clercq [23]. In order to increase the oral bioavailability for the development of oral tablets, tenofovir was subsequently developed into its oral prodrug, so-called tenofovir disoproxil (Fig. 1a) [24], [39]. In October 2001, tenofovir disoproxil fumarate (TDF) was approved by the U.S. FDA to treat HIV infections. Since then, TDF has been widely applied as an anti-HIV backbone in the highly active antiretroviral therapy (HAART) for the management of HIV infections worldwide. As of today, TDF is integrated as a key element of once-daily, fixed-dose, single-tablet regimens: Truvada®, Atripla®, Eviplera®, Stribild®, Symfi™, Symfi Lo™, Cimduo™, and Delstrigo™. In these regimens, TDF is often combined with either emtricitabine or lamivudine plus other compounds [36], [37]. However, bone and renal safety is the main concern with TDF in clinical practice [40].
Tenofovir alafenamide (GS-7340) is another prodrug of tenofovir with better antiviral activity and intracellular distribution [41]. Compared with TDF, TAF delivers a higher intracellular concentration of the active tenofovir-diphosphate into tissues of lymphatic origin, where HIV replicates [42], [43], [44]. As a result, the dose of TAF 25 mg, which is less than one-tenth of TDF 300 mg, potentially leads to the reduced risk of renal and bone toxicity in clinical use [45]. As a replacement for TDF, TAF is currently added to the fixed-dose, single-tablet regimens: Descovy®, Genvoya®, Odefsey®, Biktarvy®, and Symtuza®. A meta-analysis reveals that TAF- and TDF-containing regimens share similar tolerability, safety, and effectiveness in treatment-naïve and treatment-experienced patients infected with HIV-1 [45]. However, TAF-containing regimens showed advantages in renal functions, bone mineral density parameters, and lipid profiles [46].
In the treatment of chronic HBV infections, TDF and TAF are recommended as the preferred first-line monotherapy according to the practice guidance from the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) [47], [48]. Both TDF and TAF induce little to no risk for drug resistance mutations in HBV viruses [49]. Based on phase 3 and 4 clinical trials, Table 2 summarizes the clinical efficacy of the TDF and TAF monotherapy in treatment-naïve patients infected with HBV. Given the clinical endpoint of HBV DNA < 69 IU/mL (equivalent to 400 copies/mL) at week 48, TDF 300 mg showed significant advantages over adefovir dipivoxil 10 mg (87.5% versus 15.3% in HBeAg-positive patients, 82.3% versus 61.2% in HBeAg-negative patients). When the clinical endpoint was defined by HBV DNA < 29 IU/mL (equivalent to 169 copies/mL) at week 48, the clinical efficacy was comparable between TDF 300 mg and TAF 25 mg (67% versus 64% in HBeAg-positive patients, 93% versus 94% in HBeAg-negative patients) (Fig. 1b). According to the 108 and 110 clinical trials, TAF with better bone and renal safety could be an alternative to TDF in the treatment of HBV infections [50], [51]. A switch from TDF to TAF in virologically suppressed patients with chronic HBV is safe and effective because of the improvement of TDF-associated renal and bone abnormalities [52].
Table 2.
Clinical efficacy of TDF and TAF in phase 3 and 4 studies of treatment-naïve patients infected with HBV.
| Disease | Clinical trial | Clinical endpoint | Treatment | Clinical efficacy | Ref. |
|---|---|---|---|---|---|
| HBeAg-positive HBV | Trial 0103 (phase 3) | HBV DNA < 69 IU/mL at 48w | TDF 300 mg | 67% (177/176) | [137] |
| Adefovir 10 mg | 12% (11/90) | [137] | |||
| NCT01300234 (phase 3) | HBV DNA < 69 IU/mL at 48w | TDF 300 mg | 76.7% (79/103) | [138] | |
| Adefovir 10 mg | 18.2% (18/99) | [138] | |||
| NCT01937806 (phase 3) | HBV DNA < 69 IU/mL at 48w | TDF 300 mg | 84.9% (79/93) | [139] | |
| Besifovir 150 mg | 80.9% (76/94) | [139] | |||
| NCT00736190 (phase 4) | HBV DNA < 69 IU/mL at 48w | TDF 300 mg | 70% (37/53) | [140] | |
| Trial 110 (phase 3) | HBV DNA < 29 IU/mL at 48w | TDF 300 mg | 67% (195/292) | [50] | |
| TAF 25 mg | 64% (371/581) | [50] | |||
| HBeAg-negative HBV | Trial 0102 (phase 3) | HBV DNA < 69 IU/mL at 48w | TDF 300 mg | 71% (177/250) | [137] |
| Adefovir 10 mg | 49% (61/125) | [137] | |||
| NCT01300234 (phase 3) | HBV DNA < 69 IU/mL at 48w | TDF 300 mg | 96.8% (149/154) | [138] | |
| Adefovir 10 mg | 71.2% (109/153) | [138] | |||
| NCT00736190 (phase 4) | HBV DNA < 69 IU/mL at 48w | TDF 300 mg | 100% (37/37) | [140] | |
| Trial 108 (phase 3) | HBV DNA < 29 IU/mL at 48w | TDF 300 mg | 93% (130/140) | [51] | |
| TAF 25 mg | 94% (268/285) | [51] | |||
2.2. Valacyclovir
Valacyclovir (alternative name: valaciclovir) was first described by Leon Colla, Erik De Clercq, Roger Busson, and Hubert Vanderhaeghe in 1983 [14]. As a purine nucleoside analogue that inhibits viral DNA replication, valacyclovir was modified from acyclovir by adding the L-valyl ester (Fig. 2a) to improve the oral bioavailability of acyclovir [14]. Of note, the metabolism of acyclovir in the liver is poor and up to 90% of acyclovir is directly excreted by renal clearance with the plasma elimination half-life <5 h [53]. Acyclovir is commonly administered five times per day in an intravenous form because of its low bioavailability (15% to 30%) [54]. Intravenous formulations of acyclovir are often unavailable or unaffordable in resource-limited settings; therefore, the oral tablets of valacyclovir can be an alternative to acyclovir [55]. Despite the advantage of oral administration, the high-dose valacyclovir should be closely monitored regarding renal functions and neurological side effects [56]. In June 1995, the oral tablet of valacyclovir was approved by the U.S. FDA to treat cold sores, genital herpes, herpes zoster, and chickenpox according to the label instructions.
The efficacy and safety of valacyclovir versus acyclovir were evaluated by many randomized placebo-controlled studies. As summarized in Table 3 , valacyclovir significantly reduced the rates of HSV recurrence in patients with either recurrent cold sores [57], recurrent genital herpes [58], [59], newly diagnosed genital herpes [60], or recurrent anogenital herpes coinfected with HIV [61]. When the efficacy outcome was measured by the number of days from treatment initiation to complete cessation of all signs and symptoms (including aborted lesions), valacyclovir and acyclovir offered comparable efficacy in the treatment of herpes zoster [62], [63] and (initial or recurrent) genital herpes [64], [65]. Adverse events of valacyclovir were similar to that of acyclovir [63]. In addition to its application against HSV and VZV, valacyclovir may also have the potential to prevent or treat HCMV infections in (i) immunocompetent critically ill adults [66], (ii) kidney-pancreas EBV-seronegative kidney recipients [67], and (iii) women who wish to continue their pregnancy [68], [69]. In 2018, valacyclovir was ranked as the 140th most commonly prescribed medication with nearly five-million total prescriptions in the USA (https://clincalc.com).
Table 3.
Clinical efficacy of valaciclovir as suppressive therapy of HSV infections.
| Disease | Virus | Primary outcome | Treatment | Efficacy | Ref. |
|---|---|---|---|---|---|
| Recurrent cold sores | HSV-1 | Cold sore lesion-free for 3 weeks | Valaciclovir 2 g twice daily for 1 day | 43.8% (267/609) | [57] |
| Placebo | 36.6% (223/609) | ||||
| Recurrent genital herpes | HSV-2 | HSV recurrence-free for 16 weeks | Valaciclovir 500 mg once daily for 16w | 69% (199/288) | [58] |
| Placebo | 9.5% (9/94) | ||||
| Recurrent genital herpes | HSV-1, HSV-2 | HSV recurrence-free for 16 weeks | Valaciclovir 500 mg once daily for 16w | 72% (116/161) | [59] |
| Famciclovir 250 mg twice daily for 16w | 66% (105/159) | ||||
| Newly diagnosed genital herpes | HSV-2 | HSV recurrence-free for 24 weeks | Valaciclovir 1 g once daily for 24w | 64.7% (165/255) | [60] |
| Placebo | 46.1% (59/128) | ||||
| Genital herpes | HSV-2 | HSV transmission for 32 weeks | Valaciclovir 500 mg once daily for 32w | 1.9% (14/743) | [141] |
| Placebo | 4.2% (31/741) | ||||
| Recurrent anogenital herpes in HIV adults | HSV | HSV recurrence-free for 48 weeks | Valaciclovir 500 mg twice daily for 48w | 82% (291/355) | [61] |
| Acyclovir 400 mg twice daily for 48w | 78% (272/349) |
2.3. Brivudine (RP-101)
The first description of brivudine (Fig. 2b) as a selective antiviral agent against HSV-1 was provided by Erik De Clercq et al. [9]. The long journey of brivudine development was described by previous reviews [37], [70], [71]. As a thymidine nucleoside analogue, brivudine is phosphorylated into its 5′-triphosphate form by virus-encoded thymidine kinases and cellular kinases; subsequently, the brivudine 5′-triphosphate competes with the natural substrate of viral DNA polymerases and blocks the viral DNA replication and transcription [72]. Since the first approval in 2000, brivudine has been licensed to treat herpes zoster under different brand names such as Zostex® (Germany, Turkey, China), Zerpex® (Belgium), and Zostavir® (Czech Republic, Greece). In addition to its approved applications, brivudine has the potential to treat Epstein–Barr virus encephalitis [73], [74] and pancreatic cancer [75], but it offers little to no inhibition against HSV-2 and HCMV [70]. Importantly, brivudine should not be co-administered with 5-fluorouracil or related substances, because the degradation product of brivudine may increase the toxicity of 5-fluorouracil that causes severe symptoms [70].
Clinical efficacy and safety of brivudine have been evaluated by double-blind, randomized studies [76], [77]. In a randomized study of 2027 immunocompetent patients ≥ 50 years with herpes zoster, brivudine (125 mg, once daily) or famciclovir (250 mg, three times per day) was orally administered for 7 days and the primary outcome was defined by the postherpetic neuralgia – at least moderate pain after the treatment initiation for ≥ 3 months [76]. Brivudine and famciclovir offered similar efficacy in the treatment of herpes zoster regarding the prevalence of postherpetic neuralgia (11.1% versus 9.2%, p-value = 0.17), the duration of postherpetic neuralgia (46.5 versus 58 days, p-value = 0.54), and the time from treatment initiation to the last vesicular eruption (40.5 versus 41.0 days, p-value = 0.91) [76]. In a phase 3 study of 1227 immunocompetent adults with herpes zoster, the monotherapy of brivudine (125 mg, once daily) and acyclovir (800 mg, five times per day) was orally administered for 7 days [77]. Both the intent-to-treat and the per-protocol analyses suggested that the time to the last eruption of herpes zoster vesicles was much shorter in the brivudine group than in the acyclovir group (p-value < 0.02) [77]. In a retrospective study of 89 immunocompetent acute patients with herpes zoster, acute pain in severe cases was significantly reduced on day 3 in the brivudine group, suggesting that once-daily brivudine could be a favorable choice to control pain earlier in patients with severe herpes zoster [78].
2.4. Plerixafor (AMD3100)
Plerixafor (AMD3100) is a CXCR4 antagonist with an azamacrocycle consisting of two cyclam rings bridged by a 1,4-phenylenebis(methylene) linker (Fig. 3a). Plerixafor was initially discovered as an anti-HIV agent by Prof. Erik De Clercq et al. [28], but its HIV application was discontinued primarily due to the lack of oral bioavailability [79]. Subsequent studies suggested that plerixafor plus granulocyte-colony stimulating factor (G-CSF) enhanced the mobilization of hematopoietic stem cells to the peripheral blood for the collection of peripheral CD34 + hematopoietic cells [80], [81]. Notably, plerixafor blocks the chemokine binding pocket of CXCR4 and inhibits the binding of CXCR4 to its specific chemokine called C-X-C motif chemokine 12 (CXCL12), thereby enhancing the migration of hematopoietic stem cells to the peripheral blood [82].
On 15 December 2008, the U.S. FDA approved the clinical use of plerixafor plus G-CSF for the mobilization of hematopoietic stem cells in cancer patients with multiple myeloma or non-Hodgkin’s lymphoma [2]. According to the label instructions, cancer patients should receive G-CSF once daily for four days before apheresis sessions, and take plerixafor plus G-CSF for another four days during the peripheral stem cell collection (subcutaneous injection of plerixafor plus G-CSF should be approximately 11 h before the apheresis). The clinical efficacy of plerixafor plus G-CSF was summarized in Table 4 based on three phase 3 clinical trials [83], [84], [85]. In patients with multiple myeloma, the clinical efficacy (defined by the collection of ≥ 2 × 106 CD34 + cells/kg within 4 apheresis sessions) was higher in the plerixafor plus G-CSF group (75.7%, 112/148) than the placebo plus G-CSF group (51.3%, 79/154) [85]. In patients with non-Hodgkin’s lymphoma, the clinical efficacy (defined by the collection of ≥ 5 × 106 CD34 + cells/kg in ≤ 4 apheresis sessions) was much higher in the plerixafor plus G-CSF group (60%, 120/200) than the placebo plus G-CSF group (19.7%, 39/198) [83], [84]. Common adverse events included diarrhea, nausea, fatigue, and injection site reactions.
Table 4.
Clinical efficacy of plerixafor in cancer patients with multiple myeloma or non-Hodgkin’s lymphoma.
| Disease | Treatment | Primary outcome* | Efficacy | Phase 3 study | Ref. |
|---|---|---|---|---|---|
| Multiple myeloma | Plerixafor plus G-CSF | ≥2 × 106 CD34 + cells/kg | 141/148 (95.3%) | Study 3102 | [85] |
| ≥6 × 106 CD34 + cells/kg | 112/148 (75.7%) | ||||
| Placebo plus G-CSF | ≥2 × 106 CD34 + cells/kg | 136/154 (88.3%) | |||
| ≥6 × 106 CD34 + cells/kg | 79/154 (51.3%) | ||||
| non-Hodgkin’s lymphoma | Plerixafor plus G-CSF | ≥2 × 106 CD34 + cells/kg | 130/150 (86.7%) | Study 3101 | [83] |
| 44/50 (88%) | NCT01767714 | [84] | |||
| ≥5 × 106 CD34 + cells/kg | 89/150 (59.3%) | Study 3101 | [83] | ||
| 31/50 (62%) | NCT01767714 | [84] | |||
| Placebo plus G-CSF | ≥2 × 106 CD34 + cells/kg | 70/148 (47.3%) | Study 3101 | [83] | |
| 33/50 (66%) | NCT01767714 | [84] | |||
| ≥5 × 106 CD34 + cells/kg | 29/148 (19.6%) | Study 3101 | [83] | ||
| 10/50 (20%) | NCT01767714 | [84] | |||
*: Peripheral stem cell collection was conducted within 4 apheresis sessions.
In addition to its approved applications for multiple myeloma or non-Hodgkin’s lymphoma, plerixafor has also been considered for the treatment of many diseases such as warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome [86], glioblastoma [87], colorectal and pancreatic cancer [88], leukemia [89], and sickle cell disease [90]. Further clinical trials are needed to evaluate these new applications.
2.5. Rabacfosadine (GS-9219)
Rabacfosadine (GS-9219, VDC-1101) is the first drug approved for the treatment of canine lymphoma - one of the most common cancers in dogs. Similar to non-Hodgkin’s lymphoma in humans, canine lymphoma is a naturally occurring cancer that accounts for 7% to 24% of all canine cancers [91]. Rabacfosadine is a double prodrug of the acyclic nucleotide phosphonate of 9-(2-phosphonylmethoxyethel) guanine (PMEG, Fig. 4a). In an early study of Erik De Clercq et al. [35], PMEG was originally reported as an antiviral compound against HCMV, HSV-1, and VZV. Due to its high toxicity in humans [92], PMEG was further developed into its intracellular prodrug with a phosphonamidate moiety for the treatment of lymphoma in dogs [3], [93]. In December 2016, rabacfosadine (brand name: Tanovea®-CA1) was conditionally approved by the U.S. FDA. According to the label instructions, rabacfosadine should be administered as a 30-minute intravenous infusion at a dose of 1 mg/kg every 21 days for up to 5 doses. Adverse events can be managed by reducing doses from 1 mg/kg to 0.8 mg/kg, 0.6 mg/kg, or dose delays.
The efficacy and safety of rabacfosadine have been reported by several clinical studies in dogs [94], [95], [96], [97]. The clinical efficacy of rabacfosadine was often measured by the overall response rate of dogs experiencing either partial responses or complete responses during the post-treatment period [94], [95], [96], [97]. In the treatment of T-cell lymphoma using the 1 mg/kg dose of rabacfosadine, the clinical efficacy was 47% (8/17) and 50% (2/4) in the treatment-naive and treatment-experienced dogs, respectively (Fig. 4b). In the treatment of B-cell lymphoma using the 1 mg/kg dose of rabacfosadine, the clinical efficacy was 97% (29/30) and 77% (24/31) in the treatment-naive and treatment-experienced dogs, respectively (Table 5 ). After the treatment of rabacfosadine monotherapy, the progression-free interval was much longer in B-cell lymphoma dogs (median: 132 days) than T-cell lymphoma dogs (median: 14 days) [97]. Despite its approval to treat canine lymphoma, rabacfosadine is currently not pursued to treat human diseases.
Table 5.
Clinical efficacy of rabacfosadine in dogs.
| Disease | Treatment & | Subjects | Clinical efficacy* | Ref. |
|---|---|---|---|---|
| T-cell lymphoma | 1 mg/kg | Treatment-experienced dogs | 50% (2/4) | [95] |
| 1 mg/kg | Treatment-naive dogs | 43% (3/7) | [95] | |
| 1 mg/kg | Treatment-naive dogs | 50% (5/10) | [94] | |
| 0.82 mg/kg | Treatment-naive dogs | 50% (2/4) | [94] | |
| B-cell lymphoma | 1 mg/kg | Treatment-experienced dogs | 77% (24/31) | [96] |
| 0.82 mg/kg | Treatment-experienced dogs | 69% (11/16) | [96] | |
| 1 mg/kg | Treatment-naive dogs | 97% (29/30) | [94] | |
| 0.82 mg/kg | Treatment-naive dogs | 100% (11/11) | [94] | |
| Non-Hodgkin's lymphoma | 0.66 mg/kg | Treatment-naive dogs# | 67% (2/3) | [97] |
| 0.82 mg/kg | Treatment-naive dogs# | 50% (2/4) | [97] | |
&: A 30-minute intravenous infusion of rabacfosadine was administered once every 3 weeks for 5 doses
*: Clinical efficacy was defined by the overall response rate of dogs experiencing either partial responses or complete responses during the post-treatment period.
#: Previous treatment was allowable with ≥ three-week washouts from the most recent therapy.
3. A triangle team of chemists, virologists, and entrepreneurs for antiviral drug discovery
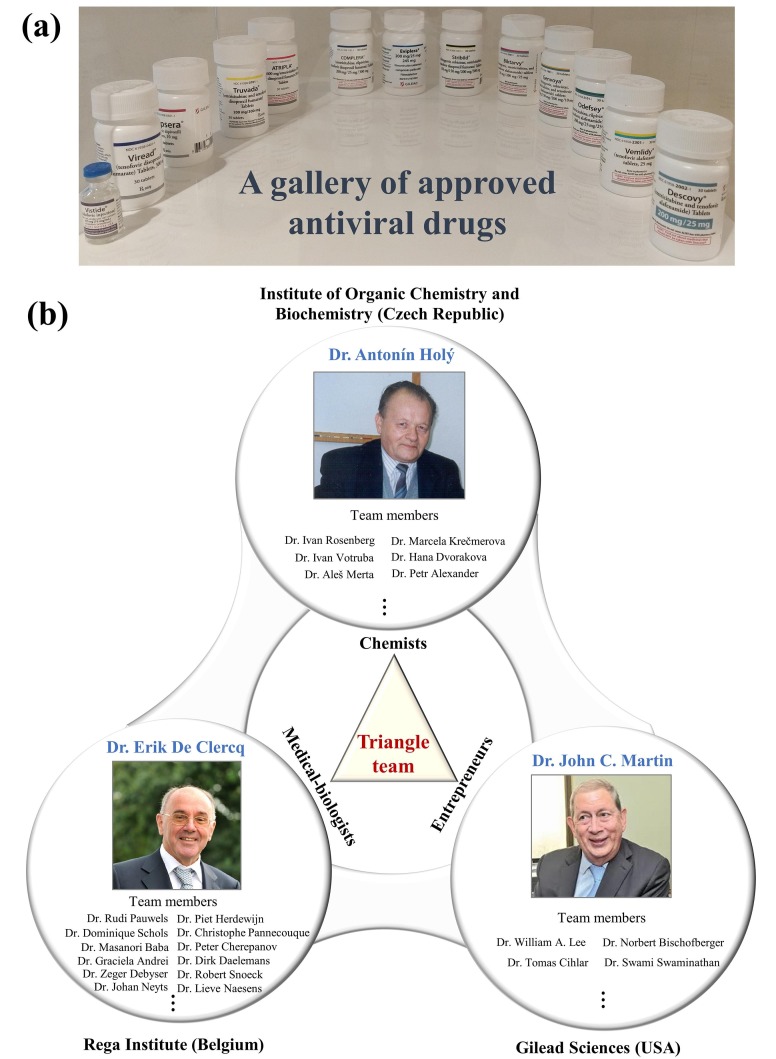
Drug discovery of nucleos(t)ide antiviral analogues is the most important scientific achievement of Prof. Erik De Clercq, which was forged by a triangle team (Fig. 5 ): Prof. Antonín Holý (a chemist), Dr. John C. Martin (an entrepreneur), and Prof. Erik De Clercq (a medical doctor and virologist) [98]. Similar to the triangle offense in NBA basketball, a triangle team is productive and stable for successful drug discovery.
Fig. 5.
Approved antiviral drugs and the triangle team. (a) A gallery of approved antiviral drugs from the left to the right: Vistide®, Viread®, Truvada®, Atripla®, Complera®, Eviplera®, Stribild®, Biktarvy®, Genvoya®, Odefsey®, Vemlidy®, and Descovy®. (b) The triangle team was forged by Dr. Antonín Holý, Dr. Erik De Clercq, and Dr. John C. Martin. Due to the limited space, we apologize for not listing all team members.
Prof. Antonín Holý, known for his extraordinary skills to synthesize nucleos(t)ide analogues, was a brilliant and hard-working chemist from the Institute of Organic Chemistry and Biochemistry (IOCB) of the Academy of Sciences of the Czech Republic. Back in the 1970s, synthesizing nucleos(t)ide analogues in Prague was quite a challenge and Prof. Antonín Holý had to milk an African snake to get fresh snake venom that could be used to extract enzymes for nucleotide analytical chemistry [98]. After their first conference meeting in 1976, Prof. Erik De Clercq sent precious reagents to Prof. Antonín Holý. In return, Prof. Antonín Holý sent new compounds to have them evaluated for their antiviral activities by the research laboratory of Prof. Erik De Clercq who, at that time, had established systems to test antiviral activities of more than 10 viruses. When their collaborations suddenly met the emergence of AIDS/HIV in 1983, their significant discoveries of nucleos(t)ide inhibitors to block HIV reverse transcriptase attracted great attention worldwide [16], [35], [99], [100], [101], [102].
Although Prof. Antonín Holý and Prof. Erik De Clercq dreamed of applying their compounds to cure HIV infections, it was beyond their budgets to buy an expensive ticket that pipelined their promising compounds into human clinical trials. The mere hope of prioritizing their compounds into human clinical trials almost faded away after many rejections from big companies such as Janssen Pharmaceutica and Bristol-Myers Squibb. The critical piece of this dream might have never been assembled without the devotion of Dr. John C. Martin – a brilliant chemist and decisive entrepreneur who is known as the former chairman and CEO of Gilead Sciences. Dr. John C. Martin earned his Ph.D. in organic chemistry from the University of Chicago, an MBA degree from Golden Gate University, and a bachelor’s degree in chemical engineering from Purdue University. As soon as the AIDS/HIV outbreak was reported in the USA, he was enthusiastic to find a potential cure. During his tireless research, Dr. John C. Martin believed in the anti-HIV nucleos(t)ide analogues from Prof. Antonín Holý and Prof. Erik De Clercq. To prioritize these compounds in human clinical trials, Dr. John C. Martin made a critical decision in 1990 to leave Bristol-Myers Squibb and to join Gilead Sciences – a small company with only 15 staff members at that time. To ensure the success of antiviral nucleotide compounds, Dr. John C. Martin persistently flew from the USA to meet Prof. Erik De Clercq in Leuven and Prof. Antonín Holý in Prague countless times during the 1980s and 1990s. Later on, their life-long friendship paved their success in the development of FDA-approved compounds including tenofovir disoproxil fumarate, tenofovir alafenamide, adefovir dipivoxil, cidofovir, and rabacfosadine, which were all licensed to Gilead Sciences.
On 16 July 2012, Prof. Antonín Holý passed away at the age of 75 years because of Parkinson's disease and its complications. However, his legacy will continue with the new generation of his young students and chemists (e.g., Dr. Tomas Cihlar, the co-developer of remdesivir and the vice president of virology at Gilead Sciences). For more than 30 years, the close friendship of Dr. John C. Martin with our esteemed professor continues and no matter how busy they are, it has become a tradition for them to meet every year. More anecdotes of the triangle team can be found in an informative book written by Renilde Loeckx [98].
4. The life-long passion for teaching and research
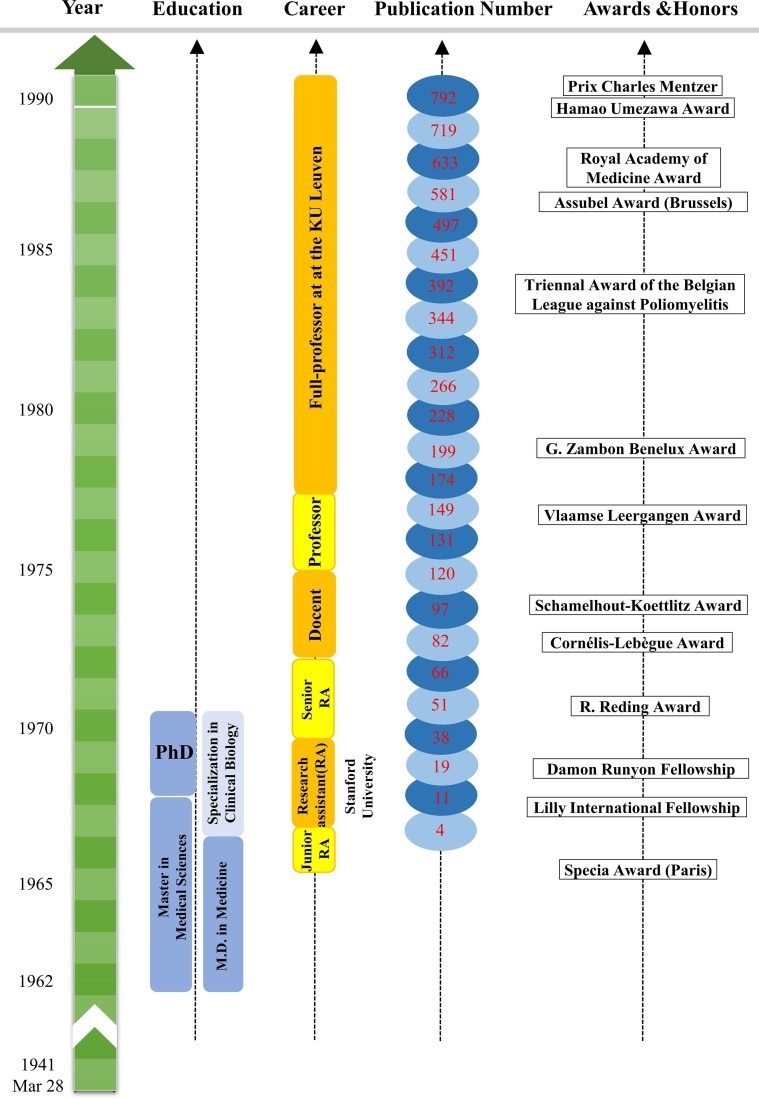
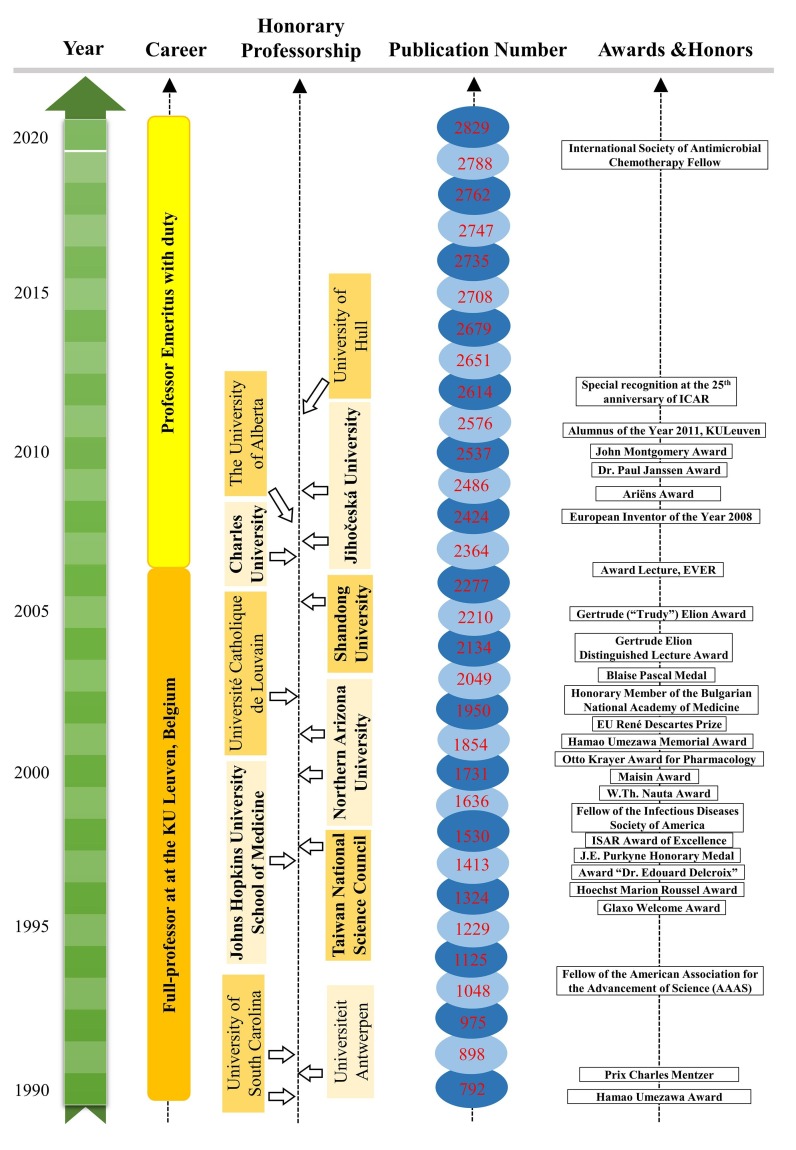
Interest is the best teacher. Here, we summarize two important interests of Prof. Erik De Clercq, teaching and research, that drive his life-long achievements in the past decades. Milestone events of Prof. Erik De Clercq are visualized by Figs. 6 and 7 .
Fig. 6.
Milestone events of Prof. Erik De Clercq from 28 March 1941 to 1990.
Fig. 7.
Milestone events of Prof. Erik De Clercq from 1990 to 2020.
4.1. Teaching
The first life-long passion of Prof. Erik De Clercq is teaching – an amazing hobby that was cultivated by family education during his teenage years. Prof. Erik was born in a middle-class family in Hamme, a village located in the East Flanders of Belgium. As a single son in the family, he received great attention from his parents. His father, Polydoor Joseph Henri De Clercq (1 November 1911 to 21 December 2001), had to work in a fertilizer factory during the day. Therefore, his mother, Celina Ludovica Van den Broeck (5 April 1912 to 8 February 1991), played a significant role in family education. In the 1940s, higher education at that time was not considered as very important as today because a university degree was not required by most jobs during and after the Second World War. Mrs. Celina, however, was an extraordinary mother who actually had an early vision of cultivating a medical doctor and strictly required her son to fully focus on the study since his first day in school. Unavoidably, her son became the first in the whole family who has ever entered the university and was always scored 1st in his class from primary school to university.
The parents’ example set up a good model during the teenage years of Prof. Erik De Clercq. As a skillful tailor, his mother trained many young apprentices (14 to 18 years old) in her dressmaker workshop on the first floor of the cramped house. Because no machine was used at that time, the manual tailoring required hard work from the early morning till the sunset. More often than not, there was no time even for a lunch break. Furthermore, teaching young apprentices requires patience and teaching skills – a gift which passed on from his mother to Erik. Although no outdoor games or parties were allowed by his mother, Erik even at the age of 5 or 6 found great amusement to teach young maids how to memorize the names of capitals from >100 countries worldwide, which somehow reflected his photographic memory. This teaching experience, which seemed an unrelated and unimportant event of Prof. Erik De Clercq in his early life, added much amusement to his “boring” study time and self-confidence to teach in front of people. In one of his publications, our revered professor made a beautiful summary of this teaching hobby as follows: “I was not born to be a researcher (or “scientist”), but born to be a teacher (or “professor”). What I learned from my parents were the virtues of hard-working, perseverance, honesty, and enthusiasm” [103].
Started as an amusement during his teenage years, teaching was later growing into a life-long hobby of Prof. Erik De Clercq. Since the beginning of his academic career, he has consistently fulfilled teaching duties at his busy or difficult times even after retirement. From 1972 to 2009, Prof. Erik De Clercq and Prof. Marcel Joniau joined their efforts to teach a course called Biochemistry to medical students at the Kortrijk campus of KU Leuven for 37 years [103]. Since 2007, his voluntary teaching of the course of “Biochemistry at the Service of Medicine” to undergraduate and graduate students from the University of South Bohemia has lasted for more than 10 years till today. Of note, this special teaching arrangement could not be managed without the support and dedication from Prof. Dr. Libor Grubhoffer. During this COVID-19 pandemic, our esteemed professor even fulfilled his teaching duty through online teaching and his teaching videos are freely shared online (www.virusface.com).
As the students of Prof. Erik De Clercq, we, fortunately, undertook his COVID-19 courses and followed his generous instructions on many studies [1], [2], [36], [104], [105], [106], [107], [108], [109], [110], [111]. During the COVID-19 pandemic, our professor and our team quickly responded to the emerging infectious disease. On 10 February 2020, we presented a detailed overview of therapeutic options against COVID-19 in Nature Reviews Drug Discovery [109]. Despite the quick spread of COVID-19 in February 2020, our professor traveled to the University of South Bohemia in České Budějovice and fulfilled his teaching duty in giving final examinations to his bachelor and master students. To prepare research articles in time, many important comments were communicated through international telephone calls. After the oral communication with our professor, we reached a common sense that human immune responses can be activated to fight against COVID-19. This idea led to our publication of IgG and IgM antibody seroconversion in asymptomatic and symptomatic patients infected with SARS-CoV-2 [107]. Given the early outbreak of COVID-19 in February 2020, our professor advised us to publish a clinical study that characterized the clinical features of older and younger patients infected with SARS-CoV-2 [108]. Based on overwhelming news and clinical experience[112], we noticed that many elderly males died of COVID-19, but the mortality causes remained unclear. With the support of our esteemed professor, we presented a multi-country survey of COVID-19 to characterize mortality-associated factors in elderly males with comorbidities [111]. In addition to remdesivir and dexamethasone, many strategies are still under development to treat COVID-19 [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127].
In the past five decades, the life-long teaching of Prof. Erik De Clercq has cultivated young generations with more than 5000 students worldwide. Perhaps, the teaching itself brings him not only the happiness to share his profound knowledge, but also the proudness to guide many of his young students who embark on a bright future and establish their reputation worldwide. The importance of teaching could be summarized by our beloved professor as follows: “teaching and research are complementary in that they cross-fertilize each other: teaching helps to resolve problems that could help in successfully planning research, and research should help formulating how to teach” [103].
4.2. Antiviral research
Antiviral research is the second life-long passion of Prof. Erik De Clercq. This passion was profoundly enriched by his mentors (Prof. Piet De Somer, Prof. Thomas Charles Merigan), collaborators (e.g., Prof. Antonín Holý, Dr. John C. Martin), and students (e.g., Dr. Rudi Pauwels, Prof. Masanori Baba, Prof. Piet Herdewijn, Prof. Peter Cherepanov, Prof. Dominique Schols, Prof. Zeger Debyser, Prof. Johan Neyts, Prof. Christophe Pannecouque, Prof. Graciela Andrei, Prof. Robert Snoeck, Prof. Lieve Naesens, and Prof. Dirk Daelemans).
Prof. Piet De Somer, an exceptional master of emotional intelligence, was a talented physician, professor, and entrepreneur who determined the career path of Prof. Erik De Clercq. Prof. Piet De Somer is known for his development of polio vaccines and the mass production of penicillin discovered during the Second World War. As soon as Prof. Piet De Somer saw the talents of his pupil Erik in the fourth year of his M.D. studies in 1963, he offered his pupil to join his team, but his offer was refused by an ambitious medical student at that time. One year later, Prof. Piet De Somer made another offer to his favored student during an oral exam when students were anxious to answer difficult questions from professors. Prof. Piet De Somer used his magic skills to persuade Erik and this time, he succeeded with an unexpected journey of professor-student collaborations for 20 years. As soon as Prof. Erik De Clercq joined his lab, Prof. Piet De Somer offered his full support. From then on, his pupil worked day and night to do research and prepared publications independently, including two in the Journal of Virology in 1967, published in 1968 [4], [5]. This was remarkable because, in the 1960s, publications were not mandatory for students to graduate and for professors to get promotions and funding. When his pupil became knowledgeable and passionate about new research areas, Prof. Piet De Somer understood his limitations in time and research experience, especially when he became the first rector (1968 to 1985) of KU Leuven after the split of the bilingual Catholic University of Leuven in 1968. For this reason, he recommended his pupil to receive systematic training in cutting-edge research in the USA. With the full support of Prof. Piet De Somer, his pupil successfully applied for Lilly International and Damon Runyon fellowships to join the research group of Prof. Thomas Charles Merigan at the Stanford Medical Center from 1968 till 1970 [71].
Prof. Thomas Charles Merigan, one of the youngest professors from Stanford University, was a talented scientist who saw the creativity of Dr. Erik De Clercq and gave him all freedom to do any research. Prof. Thomas Charles Merigan had the magic to seek the beauty of all experimental data and enthusiastically motivated his student to publish more and more [103]. With positive feedback and encouragement, Dr. Erik De Clercq worked more than 16 h per day in the lab and wrote the manuscript draft, while his newlywed wife, Lili De Nef, amazingly worked as the best team to type the draft using an old-fashioned typewriter [103]. Sometimes, a manuscript could be typed throughout the whole night. The manuscript could be presented to the desk of Prof. Thomas Charles Merigan in the early morning and mailed to a scientific journal on the same day (back in the 1960s, manuscripts were submitted by mail). As the most hard-working member of the whole team (>10 students and post-doctors), Erik and his wife Lili worked hard even at evenings, weekends, and holidays because the whole lab was fully available for him alone. Within two years, their hard work led to 22 publications (www.virusface.com), including several in Nature [128], [129], Science [130], Journal of Clinical Investigation [131], and Annual Review of Medicine[132].
At a certain point, Prof. Piet De Somer was afraid to lose his best pupil forever when he realized that Erik and his wife Lili enjoyed their life at Stanford. In 1970, he took flights to Stanford twice, and with his magic, he successfully persuaded his pupil to return home. It was quite a misery for Erik and Lili to leave Stanford, but Prof. Pieter offered all his help (technicians and sufficient funding) to make his pupil as happy as possible. With the outstanding training at Stanford and the full support of the university president, Prof. Erik De Clercq built up his lab to explore cutting-edge antiviral research and became a professor in 1975 at the age of 34 years - one of the youngest professors in the history of KU Leuven. From then on, Prof. Piet De Somer let his pupil shine and requested no first- or corresponding-author publications. Despite this, he would visit the laboratory almost every day and asked his favorite pupil about the latest research progress: “And? Did you find anything new, anything that could become a drug?” [98]. Prof. Piet De Somer was a medical doctor and entrepreneur whose interests were curing diseases by developing new drugs or vaccines, while publications, in his opinion, were only dessert recipes but not the main course. Until his last day on 17 June 1985, Prof. Piet De Somer remained the strongest supporter of our beloved professor.
5. Critical lessons learned from Prof. Erik De Clercq
Apart from his scientific achievements, many lessons could be learned from Prof. Erik De Clercq. For young researchers, here, we summarize three points: (i) hard work and self-discipline; (ii) a focus on research topics; and (iii) a good balance between research and family life.
5.1. Hard work and self-discipline
Prof. Erik De Clercq's secret to highly productive publications is hard work and a tremendous amount of self-discipline. When our professor was a student, he worked day and night in the lab and wasted no time in games or bars. When he worked as a professor for decades, he developed a habit of wakeup at 6 AM in the early morning and worked in his office till 9 PM in the evenings. Unlike most people who may enjoy the retirement life with a pension at home, our beloved professor has willingly worked as a professor emeritus with duty (no salary) by handling peer-reviewing tasks (at least one paper/day), writing single-author scientific publications, preparing lectures for students (>30 lectures/year), reading the latest scientific publications (>1000 papers/year), and responding to research collaborations worldwide. Although he was unfortunately forced to retire at the age of 65 years by the official rule in Belgium, there is no rule to stop a free mind to work hard on the eternal goal of curing infectious diseases in all human beings.
An exceptional example to show the hard work of Prof. Erik De Clercq is the reference library. While most researchers require Endnote and other software to organize references, Prof. Erik does not require any software or computers because he has built a reference library in his memory from which he can write down the exact information of references in his publications. Prof. Erik was born with a photographic memory to remember key findings of good publications that he has ever read. Most people have already retired or even have reading problems at the age of 80 years, but our revered professor keeps on reading publications and publishing single-author reviews thanks to the typing assistance of the secretary team at the Rega Institute: Christiane Callebaut (now retired), Myriam Cornelis, Inge Aerts, Chantal Biernaux, Cathy De Meyer, and Dominique Brabants. Every year, our esteemed professor reads thousands of printed papers and keeps practicing by writing several review articles. In the repository of the Rega Institute, there are more than 10 boxes of papers, documents, and correspondences written by his hands. To improve the organization of research data and documents, each project has been well organized by a book folder with the title and people’s names. In addition to his photographic memory of publications, our beloved professor remembers the people’s names and the exact date of their first meeting.
5.2. A focus on research areas
As Plato’s old saying of “Better a little which is well done, than a great deal imperfectly”, a special focus on research is another secrecy of Prof. Erik De Clercq. In the past five decades, he has been specifically focused on virology and antiviral research. The early interests of Prof. Erik were generated by his Ph.D. promoter, Prof. Piet De Somer, in the research field of interferons and viral vaccines. After 1976, his research switched to antiviral compounds such as nucleoside and nucleotide analogues in the collaboration with Dr. Antonín Holý and Dr. John C. Martin. Even though Prof. Erik De Clercq also has side projects in cancers and other areas, antiviral research has remained the major focus of his entire career. This provides a lesson for our young researchers that a special focus on one research area may lead to a profound experience and accumulated advantage.
5.3. Balance between research and family life
Success comes with a sacrifice. The entire career of Prof. Erik De Clercq has been occupied by scientific research, teaching, and administrative work since he became the director of the Rega Institute in 1986. Even though the scientific research brings him much fame and reputation, Prof. Erik De Clercq considered that the happiest moment of his entire life was the birthday of his son on 9 May 1975 and the saddest moment was the death of his wife, Lili De Nef, on 29 December 2010 due to breast cancer. Although he has enjoyed the success of developing antiviral drugs, it was a sadness for him to face the last request of his wife Lili: “You are a medical doctor who can invent drugs to treat other patients, but why cannot you treat my disease? ” In addition to the loss of Lili 10 years ago, Prof. Erik was deeply self-blamed for spending insufficient time with his single son, Dr. Rafael De Clercq, who is now a full professor of philosophy at the Lingnan University in Hong Kong. Luckily, his two beautiful grandsons, Nicolas De Clercq and Lucas De Clercq, have brought much sunshine and happiness to his life. Overall, the philosophy of “family first, work second” is another lesson from Prof. Erik De Clercq.
6. Conclusions
In the past decades, the research team of Prof. Dr. Erik De Clercq has contributed to the development of many antiviral drugs, and most of these compounds are nucleoside and nucleotide analogues against infectious diseases such as HIV, HBV, CMV, HSV, VZV, and Ebola [133], [134], [135], [136]. In addition to his scientific achievements, many useful lessons such as hard work and self-discipline, a focus on research areas, and a good balance between research and family life, could be learned from our esteemed professor. With his life-long efforts to teach >5000 students worldwide, his legacy will also continue in the new generations who can carry forward his ambitious dream of curing current and emerging infectious diseases in the future.
Funding
This work was funded by the National Nature Science Foundation of China, grant number 31871324, 81730064, 31571368; the Hunan Youth Elite Project, China, grant number 2018RS3006; the National Science and Technology Major Project, China, grant number 2018ZX10715004. The funders had no role in study design, data collection, data analysis, data interpretation or writing of the report.
CRediT authorship contribution statement
Guangdi Li: Conceptualization, Writing - original draft, Funding acquisition. Ming Xu: Data curation. Tingting Yue: Data curation. Weijie Gu: Data curation. Li Tan: Conceptualization, Writing - original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge the support of Prof. Dr. Douglas H. Thamm for sharing the clinical results of rabacfosadine from the study [94]. We appreciate the comments from Dr. Erik De Clercq, Dr. John C. Martin, and Dr. Libor Grubhoffer to improve the manuscript.
References
- 1.De Clercq E., Li G. Approved antiviral drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016;29(3):695–747. doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miao M., De Clercq E., Li G. Clinical significance of chemokine receptor antagonists. Expert Opin. Drug Metab. Toxicol. 2020;16(1):11–30. doi: 10.1080/17425255.2020.1711884. [DOI] [PubMed] [Google Scholar]
- 3.De Clercq E. Tanovea(R) for the treatment of lymphoma in dogs. Biochem. Pharmacol. 2018;154:265–269. doi: 10.1016/j.bcp.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 4.De Somer P., De Clercq E., Billiau A., Schonne E., Claesen M. Antiviral activity of polyacrylic and polymethacrylic acids. I. Mode of action in vitro. J. Virol. 1968;2(9):878–885. doi: 10.1128/jvi.2.9.878-885.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Somer P., De Clercq E., Billiau A., Schonne E., Claesen M. Antiviral activity of polyacrylic and polymethacrylic acids. II. Mode of action in vivo. J. Virol. 1968;2(9):886–893. doi: 10.1128/jvi.2.9.886-893.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Clercq E. Suramin: A potent inhibitor of the reverse transcriptase of RNA tumor viruses. Cancer Lett. 1979;8(1):9–22. doi: 10.1016/0304-3835(79)90017-x. [DOI] [PubMed] [Google Scholar]
- 7.Mitsuya H., Popovic M., Yarchoan R., Matsushita S., Gallo R.C., Broder S. Suramin protection of T cells in vitro against infectivity and cytopathic effect of HTLV-III. Science. 1984;226(4671):172–174. doi: 10.1126/science.6091268. [DOI] [PubMed] [Google Scholar]
- 8.Broder S., Collins J., Markham P., Redfield R., Hoth D., Groopman J., Gallo R., Yarchoan R., Lane H.C., Klecker R.W., Mitsuya H., Gelmann E., Resnick L., Myers C.E., Fauci A.S. Effects of suramin on HTLV-III/LAV infection presenting as Kaposi's sarcoma or AIDS-related complex: Clinical pharmacology and suppression of virus replication in vivo. Lancet. 1985;326(8456):627–630. doi: 10.1016/s0140-6736(85)90002-9. [DOI] [PubMed] [Google Scholar]
- 9.De Clercq E., Descamps J., De Somer P., Barr P.J., Jones A.S., Walker R.T. (E)-5-(2-Bromovinyl)-2'-deoxyuridine: a potent and selective anti-herpes agent. Proc. Natl. Acad. Sci. USA. 1979;76(6):2947–2951. doi: 10.1073/pnas.76.6.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Clercq E., Degreef H., Wildiers J., De Jonge G., Drochmans A., Descamps J., De Somer P. Oral (E)-5-(2-bromovinyl)-2'-deoxyuridine in severe herpes zoster. Br. Med. J. 1980;281(6249):1178. doi: 10.1136/bmj.281.6249.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Clercq E., Descamps J., De Somer P., Holý A. (S)-9-(2,3-Dihydroxypropyl)adenine: An aliphatic nucleoside analog with broad-spectrum antiviral activity. Science. 1978;200(4341):563–565. doi: 10.1126/science.200.4341.563. [DOI] [PubMed] [Google Scholar]
- 12.Derynck R., Content J., De Clercq E., Volckaert G., Tavernier J., Devos R., Fiers W. Isolation and structure of a human fibroblast interferon gene. Nature. 1980;285(5766):542–547. doi: 10.1038/285542a0. [DOI] [PubMed] [Google Scholar]
- 13.Derynck R., Remaut E., Saman E., Stanssens P., De Clercq E., Content J., Fiers W. Expression of human fibroblast interferon gene in Escherichia coli. Nature. 1980;287(5779):193–197. doi: 10.1038/287193a0. [DOI] [PubMed] [Google Scholar]
- 14.Colla L., De Clercq E., Busson R., Vanderhaeghe H. Synthesis and antiviral activity of water-soluble esters of acyclovir [9-[(2-hydroxyethoxy)methyl]guanine] J. Med. Chem. 1983;26(4):602–604. doi: 10.1021/jm00358a029. [DOI] [PubMed] [Google Scholar]
- 15.Maudgal P.C., De Clercq E., Descamps J., Missotten L. Topical treatment of experimental herpes simplex keratouveitis with 2'-O-glycylacyclovir A water-soluble ester of acyclovir. Arch. Ophthalmol. 1984;102(1):140–142. doi: 10.1001/archopht.1984.01040030118049. [DOI] [PubMed] [Google Scholar]
- 16.De Clercq E., Holý A., Rosenberg I., Sakuma T., Balzarini J., Maudgal P.C. A novel selective broad-spectrum anti-DNA virus agent. Nature. 1986;323(6087):464–467. doi: 10.1038/323464a0. [DOI] [PubMed] [Google Scholar]
- 17.Baba M., Pauwels R., Herdewijn P., De Clercq E., Desmyter J., Vandeputte M. Both 2',3'-dideoxythymidine and its 2',3'-unsaturated derivative (2',3'-dideoxythymidinene) are potent and selective inhibitors of human immunodeficiency virus replication in vitro. Biochem. Biophys. Res. Commun. 1987;142(1):128–134. doi: 10.1016/0006-291x(87)90460-8. [DOI] [PubMed] [Google Scholar]
- 18.Baba M., Tanaka H., De Clercq E., Pauwels R., Balzarini J., Schols D., Nakashima H., Perno C.F., Walker R.T., Miyasaka T. Highly specific inhibition of human immunodeficiency virus type 1 by a novel 6-substituted acyclouridine derivative. Biochem. Biophys. Res. Commun. 1989;165(3):1375–1381. doi: 10.1016/0006-291x(89)92756-3. [DOI] [PubMed] [Google Scholar]
- 19.Miyasaka T., Tanaka H., Baba M., Hayakawa H., Walker R.T., Balzarini J., De Clercq E. A novel lead for specific anti-HIV-1 agents: 1-[(2-hydroxyethoxy)methyl]-6-(phenylthio)thymine. J. Med. Chem. 1989;32(12):2507–2509. doi: 10.1021/jm00132a002. [DOI] [PubMed] [Google Scholar]
- 20.Pauwels R., Andries K., Desmyter J., Schols D., Kukla M.J., Breslin H.J., Raeymaeckers A., Van Gelder J., Woestenborghs R., Heykants J., Schellekens K., Janssen M.A.C., De Clercq E., Janssen P.A.J. Potent and selective inhibition of HIV-1 replication in vitro by a novel series of TIBO derivatives. Nature. 1990;343(6257):470–474. doi: 10.1038/343470a0. [DOI] [PubMed] [Google Scholar]
- 21.Janssen P.A., Lewi P.J., Arnold E., Daeyaert F., de Jonge M., Heeres J., Koymans L., Vinkers M., Guillemont J., Pasquier E., Kukla M., Ludovici D., Andries K., de Bethune M.P., Pauwels R., Das K., Clark A.D., Jr., Frenkel Y.V., Hughes S.H., Medaer B., De Knaep F., Bohets H., De Clerck F., Lampo A., Williams P., Stoffels P. In search of a novel anti-HIV drug: multidisciplinary coordination in the discovery of 4-[[4-[[4-[(1E)-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2- pyrimidinyl]amino]benzonitrile (R278474, rilpivirine) J. Med. Chem. 2005;48(6):1901–1909. doi: 10.1021/jm040840e. [DOI] [PubMed] [Google Scholar]
- 22.De Clercq E. Where rilpivirine meets with tenofovir, the start of a new anti-HIV drug combination era. Biochem. Pharmacol. 2012;84(3):241–248. doi: 10.1016/j.bcp.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Balzarini J., Holý A., Jindrich J., Naesens L., Snoeck R., Schols D., De Clercq E. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob. Agents Chemother. 1993;37(2):332–338. doi: 10.1128/aac.37.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naesens L., Bischofberger N., Augustijns P., Annaert P., Van den Mooter G., Arimilli M.N., Kim C.U., De Clercq E. Antiretroviral efficacy and pharmacokinetics of oral bis(isopropyloxycarbonyloxymethyl)-9-(2-phosphonylmethoxypropyl)adenine in mice. Antimicrob. Agents Chemother. 1998;42(7):1568–1573. doi: 10.1128/aac.42.7.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGuigan C., Yarnold C.J., Jones G., Velazquez S., Barucki H., Brancale A., Andrei G., Snoeck R., De Clercq E., Balzarini J. Potent and selective inhibition of varicella-zoster virus (VZV) by nucleoside analogues with an unusual bicyclic base. J. Med. Chem. 1999;42(22):4479–4484. doi: 10.1021/jm990346o. [DOI] [PubMed] [Google Scholar]
- 26.McGuigan C., Barucki H., Carangio A., Blewett S., Andrei G., Snoeck R., De Clercq E., Balzarini J., Erichsen J.T. Highly potent and selective inhibition of varicella-zoster virus by bicyclic furopyrimidine nucleosides bearing an aryl side chain. J. Med. Chem. 2000;43(26):4993–4997. doi: 10.1021/jm000210m. [DOI] [PubMed] [Google Scholar]
- 27.De Clercq E. Highly potent and selective inhibition of varicella-zoster virus replication by bicyclic furo[2,3-d]pyrimidine nucleoside analogues. Med. Res. Rev. 2003;23(3):253–274. doi: 10.1002/med.10035. [DOI] [PubMed] [Google Scholar]
- 28.De Clercq E., Yamamoto N., Pauwels R., Baba M., Schols D., Nakashima H., Balzarini J., Debyser Z., Murrer B.A., Schwartz D. Potent and selective inhibition of human immunodeficiency virus (HIV)-1 and HIV-2 replication by a class of bicyclams interacting with a viral uncoating event. Proc. Natl. Acad. Sci. USA. 1992;89(12):5286–5290. doi: 10.1073/pnas.89.12.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Clercq E., Yamamoto N., Pauwels R., Balzarini J., Witvrouw M., De Vreese K., Debyser Z., Rosenwirth B., Peichl P., Datema R. Highly potent and selective inhibition of human immunodeficiency virus by the bicyclam derivative JM3100. Antimicrob. Agents Chemother. 1994;38(4):668–674. doi: 10.1128/aac.38.4.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Clercq E. The bicyclam AMD3100 story. Nat. Rev. Drug Discov. 2003;2(7):581–587. doi: 10.1038/nrd1134. [DOI] [PubMed] [Google Scholar]
- 31.De Clercq E. The AMD3100 story: the path to the discovery of a stem cell mobilizer (Mozobil) Biochem. Pharmacol. 2009;77(11):1655–1664. doi: 10.1016/j.bcp.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 32.De Clercq E. Recent advances on the use of the CXCR4 antagonist plerixafor (AMD3100, Mozobil) and potential of other CXCR4 antagonists as stem cell mobilizers. Pharmacol. Ther. 2010;128(3):509–518. doi: 10.1016/j.pharmthera.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 33.De Clercq E. AMD3100/CXCR4 Inhibitor. Front. Immunol. 2015;6:276. doi: 10.3389/fimmu.2015.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Clercq E. Mozobil(R) (Plerixafor, AMD3100), 10 years after its approval by the US, Food and Drug Administration. Antiviral Chem. Chemother. 2019;27 doi: 10.1177/2040206619829382. 2040206619829382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Clercq E., Sakuma T., Baba M., Pauwels R., Balzarini J., Rosenberg I., Holý A. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antiviral Res. 1987;8(5–6):261–272. doi: 10.1016/s0166-3542(87)80004-9. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y., De Clercq E., Li G. Current and emerging non-nucleoside reverse transcriptase inhibitors (NNRTIs) for HIV-1 treatment. Expert Opin. Drug Metab. Toxicol. 2019;15(10):813–829. doi: 10.1080/17425255.2019.1673367. [DOI] [PubMed] [Google Scholar]
- 37.Li G., Yue T., Zhang P., Gu W., Gao L.J., Tan L. Drug discovery of Nucleos(t)ide antiviral agents: Dedicated to Prof Dr. Erik De Clercq on Occasion of His 80th Birthday. Molecules. 2021;26(4):923. doi: 10.3390/molecules26040923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heaton S.M. Harnessing host-virus evolution in antiviral therapy and immunotherapy. Clin Transl Immunol. 2019;8(7) doi: 10.1002/cti2.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robbins B.L., Srinivas R.V., Kim C., Bischofberger N., Fridland A. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob. Agents Chemother. 1998;42(3):612–617. doi: 10.1128/aac.42.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charlton M.R., Alam A., Shukla A., Dashtseren B., Lesmana C.R.A., Duger D., Payawal D.A., Duy Cuong D., Jargalsaikhan G., Cua I.H.Y., Sollano J.D., Singh K.R., Madan K., Win K.M., Kyi K.P., Tun K.S., Salih M., Rastogi M., Saraf N., Thuy P.T.T., Hien P.T.D., Gani R.A., Mohamed R., Tanwandee T., Piratvisuth T., Sukeepaisarnjaroen W., Naing W., Hashmi Z.Y. An expert review on the use of tenofovir alafenamide for the treatment of chronic hepatitis B virus infection in Asia. J. Gastroenterol. 2020;55(9):811–823. doi: 10.1007/s00535-020-01698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eisenberg E.J., He G.X., Lee W.A. Metabolism of GS-7340, a novel phenyl monophosphoramidate intracellular prodrug of PMPA, in blood. Nucleosides Nucleotides Nucleic Acids. 2001;20(4–7):1091–1098. doi: 10.1081/NCN-100002496. [DOI] [PubMed] [Google Scholar]
- 42.Lee W.A., He G.X., Eisenberg E., Cihlar T., Swaminathan S., Mulato A., Cundy K.C. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob. Agents Chemother. 2005;49(5):1898–1906. doi: 10.1128/AAC.49.5.1898-1906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birkus G., Kutty N., He G.X., Mulato A., Lee W., McDermott M., Cihlar T. Activation of 9-[(R)-2-[[(S)-[[(S)-1-(Isopropoxycarbonyl)ethyl]amino] phenoxyphosphinyl]-methoxy]propyl]adenine (GS-7340) and other tenofovir phosphonoamidate prodrugs by human proteases. Mol. Pharmacol. 2008;74(1):92–100. doi: 10.1124/mol.108.045526. [DOI] [PubMed] [Google Scholar]
- 44.De Clercq E. Antivirals: past, present and future. Biochem. Pharmacol. 2013;85(6):727–744. doi: 10.1016/j.bcp.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Wang H., Lu X., Yang X., Xu N. The efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in antiretroviral regimens for HIV-1 therapy: Meta-analysis. Medicine (Baltimore) 2016;95(41) doi: 10.1097/MD.0000000000005146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tao X., Lu Y., Zhou Y., Zhang L., Chen Y. Efficacy and safety of the regimens containing tenofovir alafenamide versus tenofovir disoproxil fumarate in fixed-dose single-tablet regimens for initial treatment of HIV-1 infection: A meta-analysis of randomized controlled trials. Int. J. Infect. Dis. 2020;93:108–117. doi: 10.1016/j.ijid.2020.01.035. [DOI] [PubMed] [Google Scholar]
- 47.European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 48.Terrault N.A., Lok A.S.F., McMahon B.J., Chang K.M., Hwang J.P., Jonas M.M., Brown R.S., Jr., Bzowej N.H., Wong J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Clercq E. Role of tenofovir alafenamide (TAF) in the treatment and prophylaxis of HIV and HBV infections. Biochem. Pharmacol. 2018;153:2–11. doi: 10.1016/j.bcp.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 50.Chan H.L., Fung S., Seto W.K., Chuang W.L., Chen C.Y., Kim H.J., Hui A.J., Janssen H.L., Chowdhury A., Tsang T.Y., Mehta R., Gane E., Flaherty J.F., Massetto B., Gaggar A., Kitrinos K.M., Lin L., Subramanian G.M., McHutchison J.G., Lim Y.S., Acharya S.K., Agarwal K. GS-US-320-0110 Investigators, Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: A randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol. Hepatol. 2016;1(3):185–195. doi: 10.1016/S2468-1253(16)30024-3. [DOI] [PubMed] [Google Scholar]
- 51.Buti M., Gane E., Seto W.K., Chan H.L., Chuang W.L., Stepanova T., Hui A.J., Lim Y.S., Mehta R., Janssen H.L., Acharya S.K., Flaherty J.F., Massetto B., Cathcart A.L., Kim K., Gaggar A., Subramanian G.M., McHutchison J.G., Pan C.Q., Brunetto M., Izumi N., Marcellin P. GS-US-320-0108 Investigators, Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: A randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol. Hepatol. 2016;1(3):196–206. doi: 10.1016/S2468-1253(16)30107-8. [DOI] [PubMed] [Google Scholar]
- 52.Lampertico P., Buti M., Fung S., Ahn S.H., Chuang W.L., Tak W.Y., Ramji A., Chen C.Y., Tam E., Bae H., Ma X., Flaherty J.F., Gaggar A., Lau A., Liu Y., Wu G., Suri V., Tan S.K., Subramanian G.M., Trinh H., Yoon S.K., Agarwal K., Lim Y.S., Chan H.L.Y. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in virologically suppressed patients with chronic hepatitis B: A randomised, double-blind, phase 3, multicentre non-inferiority study. Lancet Gastroenterol. Hepatol. 2020;5(5):441–453. doi: 10.1016/S2468-1253(19)30421-2. [DOI] [PubMed] [Google Scholar]
- 53.Abdalla S., Briand C., Oualha M., Bendavid M., Beranger A., Benaboud S., Treluyer J.M., Zheng Y., Capito C., Demir Z., Foissac F., Winter S., Gana I., Boujaafar S., Bouazza N., Hirt D. Population pharmacokinetics of intravenous and oral acyclovir and oral valacyclovir in pediatric population to optimize dosing regimens. Antimicrob. Agents Chemother. 2020;64(12):e01426–e1520. doi: 10.1128/AAC.01426-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fletcher C., Bean B. Evaluation of oral acyclovir therapy. Drug Intell. Clin. Pharm. 1985;19(7–8):518–524. doi: 10.1177/106002808501900703. [DOI] [PubMed] [Google Scholar]
- 55.Pouplin T., Pouplin J.N., Van Toi P., Lindegardh N., Rogier van Doorn H., Hien T.T., Farrar J., Torok M.E., Chau T.T. Valacyclovir for herpes simplex encephalitis. Antimicrob. Agents Chemother. 2011;55(7):3624–3626. doi: 10.1128/AAC.01023-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bodilsen J., Nielsen H., Whitley R.J. Valaciclovir therapy for herpes encephalitis: Caution advised. J. Antimicrob. Chemother. 2019;74(6):1467–1468. doi: 10.1093/jac/dky568. [DOI] [PubMed] [Google Scholar]
- 57.Spruance S.L., Jones T.M., Blatter M.M., Vargas-Cortes M., Barber J., Hill J., Goldstein D., Schultz M. High-dose, short-duration, early valacyclovir therapy for episodic treatment of cold sores: Results of two randomized, placebo-controlled, multicenter studies. Antimicrob. Agents Chemother. 2003;47(3):1072–1080. doi: 10.1128/AAC.47.3.1072-1080.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel R., Bodsworth N.J., Woolley P., Peters B., Vejlsgaard G., Saari S., Gibb A., Robinson J. Valaciclovir for the suppression of recurrent genital HSV infection: A placebo controlled study of once daily therapy, International Valaciclovir HSV Study Group. Genitourin. Med. 1997;73(2):105–109. doi: 10.1136/sti.73.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wald A., Selke S., Warren T., Aoki F.Y., Sacks S., Diaz-Mitoma F., Corey L. Comparative efficacy of famciclovir and valacyclovir for suppression of recurrent genital herpes and viral shedding. Sex. Transm. Dis. 2006;33(9):529–533. doi: 10.1097/01.olq.0000204723.15765.91. [DOI] [PubMed] [Google Scholar]
- 60.Fife K.H., Warren T.J., Justus S.E., Heitman C.K. HS2100275 Study Team, An international, randomized, double-blind, placebo-controlled, study of valacyclovir for the suppression of herpes simplex virus type 2 genital herpes in newly diagnosed patients. Sex. Transm. Dis. 2008;35(7):668–673. doi: 10.1097/OLQ.0b013e31816d1f42. [DOI] [PubMed] [Google Scholar]
- 61.Conant M.A., Schacker T.W., Murphy R.L., Gold J., Crutchfield L.T., Crooks R.J. International Valaciclovir HSV Study Group, Valaciclovir versus aciclovir for herpes simplex virus infection in HIV-infected individuals: two randomized trials. Int. J. STD AIDS. 2002;13(1):12–21. doi: 10.1258/0956462021924550. [DOI] [PubMed] [Google Scholar]
- 62.Beutner K.R., Friedman D.J., Forszpaniak C., Andersen P.L., Wood M.J. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob. Agents Chemother. 1995;39(7):1546–1553. doi: 10.1128/aac.39.7.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schuster A.K., Harder B.C., Schlichtenbrede F.C., Jarczok M.N., Tesarz J. Valacyclovir versus acyclovir for the treatment of herpes zoster ophthalmicus in immunocompetent patients. Cochrane Database Syst. Rev. 2016;11:CD011503. doi: 10.1002/14651858.CD011503.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fife K.H., Barbarash R.A., Rudolph T., Degregorio B., Roth R. The Valaciclovir International Herpes Simplex Virus Study Group, Valaciclovir versus acyclovir in the treatment of first-episode genital herpes infection Results of an international, multicenter, double-blind, randomized clinical trial. Sex Transm. Dis. 1997;24(8):481–486. doi: 10.1097/00007435-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Bodsworth N.J., Crooks R.J., Borelli S., Vejlsgaard G., Paavonen J., Worm A.M., Uexkull N., Esmann J., Strand A., Ingamells A.J., Gibb A. Valaciclovir versus aciclovir in patient initiated treatment of recurrent genital herpes: A randomised, double blind clinical trial. Genitourin. Med. 1997;73(2):110–116. doi: 10.1136/sti.73.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cowley N.J., Owen A., Shiels S.C., Millar J., Woolley R., Ives N., Osman H., Moss P., Bion J.F. Safety and Efficacy of antiviral therapy for prevention of cytomegalovirus reactivation in immunocompetent critically Ill Patients: A Randomized Clinical Trial. JAMA Intern. Med. 2017;177(6):774–783. doi: 10.1001/jamainternmed.2017.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ville S., Imbert-Marcille B.M., Coste-Burel M., Garandeau C., Meurette A., Cantarovitch D., Giral M., Hourmant M., Blancho G., Dantal J. Impact of antiviral prophylaxis in adults Epstein-Barr Virus-seronegative kidney recipients on early and late post-transplantation lymphoproliferative disorder onset: A retrospective cohort study. Transpl. Int. 2018;31(5):484–494. doi: 10.1111/tri.13085. [DOI] [PubMed] [Google Scholar]
- 68.Zammarchi L., Lazzarotto T., Andreoni M., Campolmi I., Pasquini L., Di Tommaso M., Simonazzi G., Tomasoni L.R., Castelli F., Galli L., Borchi B., Clerici P., Bartoloni A., Tavio M., Trotta M. Management of cytomegalovirus infection in pregnancy: is it time for valacyclovir? Clin. Microbiol. Infect. 2020;26(9):1151–1154. doi: 10.1016/j.cmi.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 69.Shahar-Nissan K., Pardo J., Peled O., Krause I., Bilavsky E., Wiznitzer A., Hadar E., Amir J. Valaciclovir to prevent vertical transmission of cytomegalovirus after maternal primary infection during pregnancy: A randomised, double-blind, placebo-controlled trial. Lancet. 2020;396(10253):779–785. doi: 10.1016/S0140-6736(20)31868-7. [DOI] [PubMed] [Google Scholar]
- 70.De Clercq E. Discovery and development of BVDU (brivudin) as a therapeutic for the treatment of herpes zoster. Biochem. Pharmacol. 2004;68(12):2301–2315. doi: 10.1016/j.bcp.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 71.De Clercq E. A 40-year journey in search of selective antiviral chemotherapy. Annu. Rev. Pharmacol. Toxicol. 2011;51:1–24. doi: 10.1146/annurev-pharmtox-010510-100228. [DOI] [PubMed] [Google Scholar]
- 72.Allaudeen H.S., Kozarich J.W., Bertino J.R., De Clercq E. On the mechanism of selective inhibition of herpesvirus replication by (E)-5-(2-bromovinyl)-2'-deoxyuridine. Proc. Natl. Acad. Sci. USA. 1981;78(5):2698–2702. doi: 10.1073/pnas.78.5.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lahmer T., Hoffmann D., Heemann U., Kuchle C., Frank H. Epstein-Barr virus encephalitis after kidney transplantation and successful treatment with brivudine. Transpl. Int. 2010;23(6):e24–e25. doi: 10.1111/j.1432-2277.2009.01045.x. [DOI] [PubMed] [Google Scholar]
- 74.Pender M.P. Hypothesis: bipolar disorder is an Epstein-Barr virus-driven chronic autoimmune disease – Implications for immunotherapy. Clin. Transl. Immunol. 2020;9(4) doi: 10.1002/cti2.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heinrich J.C., Tuukkanen A., Schroeder M., Fahrig T., Fahrig R. RP101 (brivudine) binds to heat shock protein HSP27 (HSPB1) and enhances survival in animals and pancreatic cancer patients. J. Cancer Res. Clin. Oncol. 2011;137(9):1349–1361. doi: 10.1007/s00432-011-1005-1. [DOI] [PubMed] [Google Scholar]
- 76.Wassilew S. Collaborative Brivudin PHN Study Group, Brivudin compared with famciclovir in the treatment of herpes zoster: effects in acute disease and chronic pain in immunocompetent patients A randomized, double-blind, multinational study. J. Eur. Acad. Dermatol. Venereol. 2005;19(1):47–55. doi: 10.1111/j.1468-3083.2004.01119.x. [DOI] [PubMed] [Google Scholar]
- 77.Wassilew S.W., Wutzler P. Brivudin Herpes Zoster Study Group, Oral brivudin in comparison with acyclovir for improved therapy of herpes zoster in immunocompetent patients: results of a randomized, double-blind, multicentered study. Antiviral Res. 2003;59(1):49–56. doi: 10.1016/s0166-3542(03)00065-2. [DOI] [PubMed] [Google Scholar]
- 78.Yaldiz M., Solak B., Kara R.O., Cosansu N., Erdem M.T. Comparison of famciclovir, valaciclovir, and brivudine treatments in adult immunocompetent patients with herpes zoster. Am. J .Ther. 2018;25(6):e626–e634. doi: 10.1097/MJT.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 79.Hendrix C.W., Collier A.C., Lederman M.M., Schols D., Pollard R.B., Brown S., Jackson J.B., Coombs R.W., Glesby M.J., Flexner C.W., Bridger G.J., Badel K., MacFarland R.T., Henson G.W., Calandra G. AMD3100 HIV Study Group, Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV-1 infection. J. Acquir. Immune Defic. Syndr. 2004;37(2):1253–1262. doi: 10.1097/01.qai.0000137371.80695.ef. [DOI] [PubMed] [Google Scholar]
- 80.Liles W.C., Broxmeyer H.E., Rodger E., Wood B., Hubel K., Cooper S., Hangoc G., Bridger G.J., Henson G.W., Calandra G., Dale D.C. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102(8):2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 81.Liles W.C., Rodger E., Broxmeyer H.E., Dehner C., Badel K., Calandra G., Christensen J., Wood B., Price T.H., Dale D.C. Augmented mobilization and collection of CD34+ hematopoietic cells from normal human volunteers stimulated with granulocyte-colony-stimulating factor by single-dose administration of AMD3100, a CXCR4 antagonist. Transfusion. 2005;45(3):295–300. doi: 10.1111/j.1537-2995.2005.04222.x. [DOI] [PubMed] [Google Scholar]
- 82.Devine S.M., Vij R., Rettig M., Todt L., McGlauchlen K., Fisher N., Devine H., Link D.C., Calandra G., Bridger G., Westervelt P., Dipersio J.F. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood. 2008;112(4):990–998. doi: 10.1182/blood-2007-12-130179. [DOI] [PubMed] [Google Scholar]
- 83.DiPersio J.F., Micallef I.N., Stiff P.J., Bolwell B.J., Maziarz R.T., Jacobsen E., Nademanee A., McCarty J., Bridger G., Calandra G. 3101 Investigators, Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J. Clin. Oncol. 2009;27(28):4767–4773. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 84.Zhu J., Huang H., Chen H., Zhang X., Li Z., Wu D., Zhou D., Song Y., Hu Y., Liang Y., Ren H., Huang H., Li N., Chen H., Hu J., Li J., Meng R., Wu J., Yu D., Huang X. Plerixafor and granulocyte-colony-stimulating factor for mobilization of hematopoietic stem cells for autologous transplantation in Chinese patients with non-Hodgkin's lymphoma: a randomized Phase 3 study. Transfusion. 2018;58(1):81–87. doi: 10.1111/trf.14426. [DOI] [PubMed] [Google Scholar]
- 85.DiPersio J.F., Stadtmauer E.A., Nademanee A., Micallef I.N., Stiff P.J., Kaufman J.L., Maziarz R.T., Hosing C., Fruehauf S., Horwitz M., Cooper D., Bridger G., Calandra G. 3102 Investigators, Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 86.McDermott D.H., Pastrana D.V., Calvo K.R., Pittaluga S., Velez D., Cho E., Liu Q., Trout H.H., 3rd, Neves J.F., Gardner P.J., Bianchi D.A., Blair E.A., Landon E.M., Silva S.L., Buck C.B., Murphy P.M. Plerixafor for the Treatment of WHIM Syndrome. N. Engl. J. Med. 2019;380(2):163–170. doi: 10.1056/NEJMoa1808575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thomas R.P., Nagpal S., Iv M., Soltys S.G., Bertrand S., Pelpola J.S., Ball R., Yang J., Sundaram V., Lavezo J., Born D., Vogel H., Brown J.M., Recht L.D. Macrophage Exclusion after Radiation Therapy (MERT): A First in Human Phase I/II Trial using a CXCR4 Inhibitor in Glioblastoma. Clin. Cancer Res. 2019;25(23):6948–6957. doi: 10.1158/1078-0432.CCR-19-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Biasci D., Smoragiewicz M., Connell C.M., Wang Z., Gao Y., Thaventhiran J.E.D., Basu B., Magiera L., Johnson T.I., Bax L., Gopinathan A., Isherwood C., Gallagher F.A., Pawula M., Hudecova I., Gale D., Rosenfeld N., Barmpounakis P., Popa E.C., Brais R., Godfrey E., Mir F., Richards F.M., Fearon D.T., Janowitz T., Jodrell D.I. CXCR4 inhibition in human pancreatic and colorectal cancers induces an integrated immune response. Proc. Natl. Acad. Sci. USA. 2020;117(46):28960–28970. doi: 10.1073/pnas.2013644117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Borthakur G., Zeng Z., Cortes J.E., Chen H.C., Huang X., Konopleva M., Ravandi F., Kadia T., Patel K.P., Daver N., Kelly M.A., McQueen T., Wang R.Y., Kantarjian H., Andreeff M. Phase 1 study of combinatorial sorafenib, G-CSF, and plerixafor treatment in relapsed/refractory, FLT3-ITD-mutated acute myelogenous leukemia patients. Am. J. Hematol. 2020;95(11):1296–1303. doi: 10.1002/ajh.25943. [DOI] [PubMed] [Google Scholar]
- 90.Lagresle-Peyrou C., Lefrere F., Magrin E., Ribeil J.A., Romano O., Weber L., Magnani A., Sadek H., Plantier C., Gabrion A., Ternaux B., Felix T., Couzin C., Stanislas A., Treluyer J.M., Lamhaut L., Joseph L., Delville M., Miccio A., Andre-Schmutz I., Cavazzana M. Plerixafor enables safe, rapid, efficient mobilization of hematopoietic stem cells in sickle cell disease patients after exchange transfusion. Haematologica. 2018;103(5):778–786. doi: 10.3324/haematol.2017.184788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu W., Selcuk F., Rutgen B.C., Moulay M., Willenbrock S., Hammer S.E., Sterenczak K.A., Junghanss C., Hewicker-Trautwein M., Nolte I., Murua Escobar H. Evaluation of stem cell marker expression in canine B-Cell Lymphoma Cell Lines, B-Cell Lymphoma-generated Spheres and Primary Samples. Anticancer Res. 2015;35(5):2805–2816. [PubMed] [Google Scholar]
- 92.Kramata P., Downey K.M., Paborsky L.R. Incorporation and excision of 9-(2-phosphonylmethoxyethyl)guanine (PMEG) by DNA polymerase delta and epsilon in vitro. J. Biol. Chem. 1998;273(34):21966–21971. doi: 10.1074/jbc.273.34.21966. [DOI] [PubMed] [Google Scholar]
- 93.Naesens L., Hatse S., Segers C., Verbeken E., De Clercq E., Waer M., Balzarini J. 9-(2-phosphonylmethoxyethyl)-N6-cyclopropyl-2,6-diaminopurine: a novel prodrug of 9-(2-phosphonylmethoxyethyl)guanine with improved antitumor efficacy and selectivity in choriocarcinoma-bearing rats. Oncol. Res. 1999;11(4):195–203. [PubMed] [Google Scholar]
- 94.Saba C.F., Clifford C., Burgess K., Phillips B., Vail D., Wright Z., Curran K., Fan T., Elmslie R., Post G., Thamm D. Rabacfosadine for naive canine intermediate to large cell lymphoma: Efficacy and adverse event profile across three prospective clinical trials. Vet. Comp. Oncol. 2020;18(4):763–769. doi: 10.1111/vco.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morges M.A., Burton J.H., Saba C.F., Vail D.M., Burgess K.E., Thamm D.H. Phase II evaluation of VDC-1101 in canine cutaneous T-cell lymphoma. J. Vet. Intern. Med. 2014;28(5):1569–1574. doi: 10.1111/jvim.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saba C.F., Vickery K.R., Clifford C.A., Burgess K.E., Phillips B., Vail D.M., Wright Z.M., Morges M.A., Fan T.M., Thamm D.H. Rabacfosadine for relapsed canine B-cell lymphoma: Efficacy and adverse event profiles of 2 different doses. Vet. Comp. Oncol. 2018;16(1):E76–E82. doi: 10.1111/vco.12337. [DOI] [PubMed] [Google Scholar]
- 97.Vail D.M., Thamm D.H., Reiser H., Ray A.S., Wolfgang G.H., Watkins W.J., Babusis D., Henne I.N., Hawkins M.J., Kurzman I.D., Jeraj R., Vanderhoek M., Plaza S., Anderson C., Wessel M.A., Robat C., Lawrence J., Tumas D.B. Assessment of GS-9219 in a pet dog model of non-Hodgkin's lymphoma. Clin. Cancer Res. 2009;15(10):3503–3510. doi: 10.1158/1078-0432.CCR-08-3113. [DOI] [PubMed] [Google Scholar]
- 98.Loeckx R. Leuven University Press; Leuven: 2017. Cold War Triangle: How scientists in East and West tamed HIV. [Google Scholar]
- 99.Pauwels R., Balzarini J., Schols D., Baba M., Desmyter J., Rosenberg I., Holý A., De Clercq E. Phosphonylmethoxyethyl purine derivatives, a new class of anti-human immunodeficiency virus agents. Antimicrob. Agents Chemother. 1988;32(7):1025–1030. doi: 10.1128/aac.32.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Snoeck R., Sakuma T., De Clercq E., Rosenberg I., Holý A. (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob. Agents Chemother. 1988;32(12):1839–1844. doi: 10.1128/aac.32.12.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Balzarini J., Naesens L., Herdewijn P., Rosenberg I., Holý A., Pauwels R., Baba M., Johns D.G., De Clercq E. Marked in vivo antiretrovirus activity of 9-(2-phosphonylmethoxyethyl)adenine, a selective anti-human immunodeficiency virus agent. Proc. Natl. Acad. Sci. USA. 1989;86(1):332–336. doi: 10.1073/pnas.86.1.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Balzarini J., Naesens L., Slachmuylders J., Niphuis H., Rosenberg I., Holý A., Schellekens H., De Clercq E. 9-(2-Phosphonylmethoxyethyl)adenine (PMEA) effectively inhibits retrovirus replication in vitro and simian immunodeficiency virus infection in rhesus monkeys. AIDS. 1991;5(1):21–28. doi: 10.1097/00002030-199101000-00003. [DOI] [PubMed] [Google Scholar]
- 103.De Clercq E. An Odyssey in antiviral drug development-50 years at the Rega Institute: 1964–2014. Acta Pharm Sin B. 2015;5(6):520–543. doi: 10.1016/j.apsb.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miao M., Jing X., De Clercq E., Li G. Danoprevir for the Treatment of Hepatitis C Virus Infection: Design Development, and Place in Therapy. Drug Des. Devel Ther. 2020;14:2759–2774. doi: 10.2147/DDDT.S254754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li G., De Clercq E. HIV Genome-Wide Protein Associations: A Review of 30 Years of Research. Microbiol. Mol. Biol. Rev. 2016;80(3):679–731. doi: 10.1128/MMBR.00065-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang M., Li G., Shang J., Pan C., Zhang M., Yin Z., Xie Q., Peng Y., Mao Q., Xiao X., Jiang Y., Luo K., Xu Y., Ding H., Fan W., Diego V., Pourkarim M.R., De Clercq E., Wang G., Gong G. Rapidly decreased HBV RNA predicts responses of pegylated interferons in HBeAg-positive patients: A longitudinal cohort study. Hep. Intl. 2020;14(2):212–224. doi: 10.1007/s12072-020-10015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang C., Wang Y., Hu M., Wen L., Wen C., Wang Y., Zhu W., Tai S., Jiang Z., Xiao K., Faria N.R., De Clercq E., Xu J., Li G. Antibody seroconversion in asymptomatic and symptomatic patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Transl Immunol. 2020;9(9) doi: 10.1002/cti2.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou Z., Zhang M., Wang Y., Zheng F., Huang Y., Huang K., Yu Q., Cai C., Chen D., Tian Y., Lei J., Xiao X., De Clercq E., Li G., Xie Y., Gong G. Clinical characteristics of older and younger patients infected with SARS-CoV-2. Aging. 2020;12(12):11296–11305. doi: 10.18632/aging.103535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 110.Li G., De Clercq E. Current therapy for chronic hepatitis C: The role of direct-acting antivirals. Antiviral Res. 2017;142:83–122. doi: 10.1016/j.antiviral.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li G., Liu Y., Jing X., Wang Y., Miao M., Tao L., Zhou Z., Xie Y., Huang Y., Lei J., Gong G., Jin P., Hao Y., Faria N.R., De Clercq E., Zhang M. Mortality risk of COVID-19 in elderly males with comorbidities: A multi-country study. Aging. 2020;13(1):27–60. doi: 10.18632/aging.202456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu L., O'Kane A.M., Peng H., Bi Y., Motriuk-Smith D., Ren J. SARS-CoV-2 and cardiovascular complications: From molecular mechanisms to pharmaceutical management. Biochem. Pharmacol. 2020;178 doi: 10.1016/j.bcp.2020.114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lisi L., Lacal P.M., Barbaccia M.L., Graziani G. Approaching coronavirus disease 2019: Mechanisms of action of repurposed drugs with potential activity against SARS-CoV-2. Biochem. Pharmacol. 2020;180 doi: 10.1016/j.bcp.2020.114169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gioia M., Ciaccio C., Calligari P., De Simone G., Sbardella D., Tundo G., Fasciglione G.F., Di Masi A., Di Pierro D., Bocedi A., Ascenzi P., Coletta M. Role of proteolytic enzymes in the COVID-19 infection and promising therapeutic approaches. Biochem. Pharmacol. 2020;182 doi: 10.1016/j.bcp.2020.114225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yousefi H., Mashouri L., Okpechi S.C., Alahari N., Alahari S.K. Repurposing existing drugs for the treatment of COVID-19/SARS-CoV-2 infection: A review describing drug mechanisms of action. Biochem. Pharmacol. 2021;183 doi: 10.1016/j.bcp.2020.114296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carty M., Guy C., Bowie A.G. Detection of viral infections by innate immunity. Biochem. Pharmacol. 2021;183 doi: 10.1016/j.bcp.2020.114316. [DOI] [PubMed] [Google Scholar]
- 117.Abdelnabi R., Jacobs S., Delang L., Neyts J. Antiviral drug discovery against arthritogenic alphaviruses: Tools and molecular targets. Biochem. Pharmacol. 2020;174 doi: 10.1016/j.bcp.2019.113777. [DOI] [PubMed] [Google Scholar]
- 118.Nguyen T.H., McAuley J.L., Kim Y., Zheng M.Z., Gherardin N.A., Godfrey D.I., Purcell D.F., Sullivan L.C., Westall G.P., Reading P.C., Kedzierska K., Wakim L.M. Influenza, but not SARS-CoV-2, infection induces a rapid interferon response that wanes with age and diminished tissue-resident memory CD8(+) T cells. Clin. Transl. Immunol. 2021;10(1) doi: 10.1002/cti2.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sohrabi Y., Dos Santos J.C., Dorenkamp M., Findeisen H., Godfrey R., Netea M.G., Joosten L.A. Trained immunity as a novel approach against COVID-19 with a focus on Bacillus Calmette-Guerin vaccine: Mechanisms, challenges and perspectives. Clin. Transl. Immunol. 2020;9(12) doi: 10.1002/cti2.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garcia M., Kokkinou E., Carrasco Garcia A., Parrot T., Palma Medina L.M., Maleki K.T., Christ W., Varnaite R., Filipovic I., Ljunggren H.G., Bjorkstrom N.K., Folkesson E., Rooyackers O., Eriksson L.I., Sonnerborg A., Aleman S., Stralin K., Gredmark-Russ S., Klingstrom J., Mjosberg J. The Karolinska KI/K COVID-19 Study Group, Innate lymphoid cell composition associates with COVID-19 disease severity. Clin Transl Immunol. 2020;9(12) doi: 10.1002/cti2.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lineburg K.E., Srihari S., Altaf M., Swaminathan S., Panikkar A., Raju J., Crooks P., Ambalathingal G.R., Martins J.P., Matthews K.K., Neller M.A., Khanna R., Smith C. Rapid detection of SARS-CoV-2-specific memory T-cell immunity in recovered COVID-19 cases. Clin. Transl. Immunol. 2020;9(12) doi: 10.1002/cti2.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Caballero A., Filgueira L.M., Betancourt J., Sanchez N., Hidalgo C., Ramirez A., Martinez A., Despaigne R.E., Escalona A., Diaz H., Merino E., Ortega L.M., Castillo U., Ramos M., Saavedra D., Garcia Y., Lorenzo G., Cepeda M., Arencibia M., Cabrera L., Domecq M., Estevez D., Valenzuela C., Lorenzo P., Sanchez L., Mazorra Z., Leon K., Crombet T. Treatment of COVID-19 patients with the anti-CD6 antibody itolizumab. Clin. Transl. Immunol. 2020;9(11) doi: 10.1002/cti2.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Galeotti C., Kaveri S.V., Bayry J. Intravenous immunoglobulin immunotherapy for coronavirus disease-19 (COVID-19) Clin. Transl. Immunol. 2020;9(10) doi: 10.1002/cti2.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang C.W., Hsu H.Y., Chang H.Y., Lee Y.Z., Lee S.J. Natural cardenolides suppress coronaviral replication by downregulating JAK1 via a Na(+)/K(+)-ATPase independent proteolysis. Biochem. Pharmacol. 2020;180 doi: 10.1016/j.bcp.2020.114122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chakraborty R., Parvez S. COVID-19: An overview of the current pharmacological interventions, vaccines, and clinical trials. Biochem. Pharmacol. 2020;180 doi: 10.1016/j.bcp.2020.114184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mohammed El Tabaa M., Mohammed El Tabaa M. Targeting Neprilysin (NEP) pathways: A potential new hope to defeat COVID-19 ghost. Biochem. Pharmacol. 2020;178 doi: 10.1016/j.bcp.2020.114057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Neufurth M., Wang X., Tolba E., Lieberwirth I., Wang S., Schroder H.C., Muller W.E.G. The inorganic polymer, polyphosphate, blocks binding of SARS-CoV-2 spike protein to ACE2 receptor at physiological concentrations. Biochem. Pharmacol. 2020;182 doi: 10.1016/j.bcp.2020.114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.De Clercq E., Merigan T.C. Requirement of a stable secondary structure for the antiviral activity of polynucleotides. Nature. 1969;222:1148–1152. doi: 10.1038/2221148a0. [DOI] [PubMed] [Google Scholar]
- 129.De Clercq E., Wells R.D., Merigan T.C. Increase in antiviral activity of polynucleotides by thermal activation. Nature. 1969;226:364–366. doi: 10.1038/226364a0. [DOI] [PubMed] [Google Scholar]
- 130.De Clercq E., Eckstein F., Merigan T.C. Interferon induction increased through chemical modification of a synthetic polyribonucleotide. Science. 1969;165:1137–1139. doi: 10.1126/science.165.3898.1137. [DOI] [PubMed] [Google Scholar]
- 131.De Clercq E., Nuwer M.R., Merigan T.C. The role of interferon in the protective effect of a synthetic double-stranded polyribonucleotide against intranasal vesicular stomatitis virus challenge in mice. J. Clin. Invest. 1970;49:1565–1577. doi: 10.1172/JCI106374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.De Clercq E., Merigan T.C. Current concepts of interferon and interferon induction. Annu. Rev. Med. 1970;21:17–46. doi: 10.1146/annurev.me.21.020170.000313. [DOI] [PubMed] [Google Scholar]
- 133.Jansen D.T., Dou Y., de Wilde J.W., Woltman A.M., Buschow S.I. Designing the next-generation therapeutic vaccines to cure chronic hepatitis B: Focus on antigen presentation, vaccine properties and effect measures. Clin. Transl. Immunol. 2021;10(1) doi: 10.1002/cti2.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McGuire H.M., Rizzetto S., Withers B.P., Clancy L.E., Avdic S., Stern L., Patrick E., Fazekas de St Groth B., Slobedman B., Gottlieb D.J., Luciani F., Blyth E. Mass cytometry reveals immune signatures associated with cytomegalovirus (CMV) control in recipients of allogeneic haemopoietic stem cell transplant and CMV-specific T cells. Clin. Transl. Immunol. 2020;9(7) doi: 10.1002/cti2.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Karbanova S., Cerveny L., Jiraskova L., Karahoda R., Ceckova M., Ptackova Z., Staud F. Transport of ribavirin across the rat and human placental barrier: Roles of nucleoside and ATP-binding cassette drug efflux transporters. Biochem. Pharmacol. 2019;163:60–70. doi: 10.1016/j.bcp.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 136.Lopez-Huertas M.R., Jimenez-Tormo L., Madrid-Elena N., Gutierrez C., Vivancos M.J., Luna L., Moreno S. Maraviroc reactivates HIV with potency similar to that of other latency reversing drugs without inducing toxicity in CD8 T cells. Biochem. Pharmacol. 2020;182 doi: 10.1016/j.bcp.2020.114231. [DOI] [PubMed] [Google Scholar]
- 137.Marcellin P., Heathcote E.J., Buti M., Gane E., de Man R.A., Krastev Z., Germanidis G., Lee S.S., Flisiak R., Kaita K., Manns M., Kotzev I., Tchernev K., Buggisch P., Weilert F., Kurdas O.O., Shiffman M.L., Trinh H., Washington M.K., Sorbel J., Anderson J., Snow-Lampart A., Mondou E., Quinn J., Rousseau F. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N. Engl. J. Med. 2008;359(23):2442–2455. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 138.Hou J.L., Gao Z.L., Xie Q., Zhang J.M., Sheng J.F., Cheng J., Chen C.W., Mao Q., Zhao W., Ren H., Tan D.M., Niu J.Q., Chen S.J., Pan C., Tang H., Wang H., Mao Y.M., Jia J.D., Ning Q., Xu M., Wu S.M., Li J., Zhang X.X., Ji Y., Dong J., Li J. Tenofovir disoproxil fumarate vs adefovir dipivoxil in Chinese patients with chronic hepatitis B after 48 weeks: a randomized controlled trial. J. Viral Hepat. 2015;22(2):85–93. doi: 10.1111/jvh.12313. [DOI] [PubMed] [Google Scholar]
- 139.Ahn S.H., Kim W., Jung Y.K., Yang J.M., Jang J.Y., Kweon Y.O., Cho Y.K., Kim Y.J., Hong G.Y., Kim D.J., Um S.H., Sohn J.H., Lee J.W., Park S.J., Lee B.S., Kim J.H., Kim H.S., Yoon S.K., Kim M.Y., Yim H.J., Lee K.S., Lim Y.S., Lee W.S., Park N.H., Jin S.Y., Kim K.H., Choi W., Han K.H. Efficacy and safety of besifovir dipivoxil maleate compared with tenofovir disoproxil fumarate in treatment of chronic hepatitis B virus infection. Clin. Gastroenterol. Hepatol. 2019;17(9):1850–1859.e4. doi: 10.1016/j.cgh.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 140.Pan C.Q., Trinh H., Yao A., Bae H., Lou L., Chan S. Efficacy and safety of tenofovir disoproxil fumarate in Asian-Americans with chronic hepatitis B in community settings. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0089789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Corey L., Wald A., Patel R., Sacks S.L., Tyring S.K., Warren T., Douglas J.M., Jr., Paavonen J., Morrow R.A., Beutner K.R., Stratchounsky L.S., Mertz G., Keene O.N., Watson H.A., Tait D., Vargas-Cortes M. Valacyclovir HSV Transmission Study Group, Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N. Engl. J. Med. 2004;350(1):11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]