Figure 1.
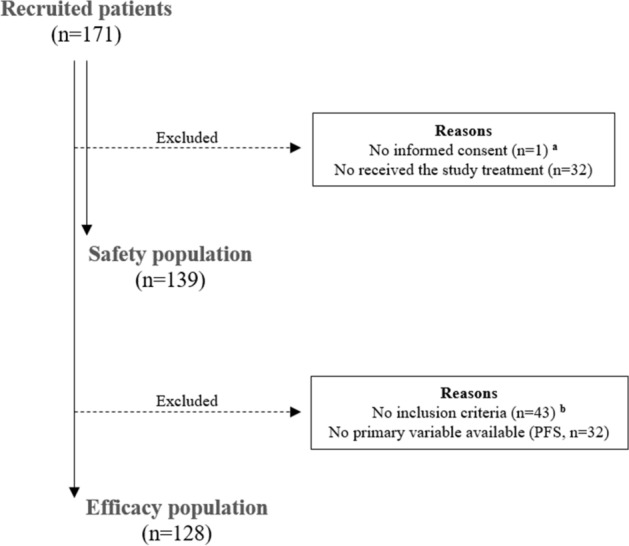
Patient flowchart. (a) Informed consent written and signed prior to any specific study of procedures. In the case of patients who died at the time of inclusion, there were no signed informed consent, so the researcher assumed responsibility for data protection and confidentiality when recording the data; (b) Patients who experienced relapse during or after the end of adjuvant anti-estrogenic therapy and received first-line treatment with Fulvestrant 500 mg per month during the study period (started treatment with Fulvestrant January 1, 2011 to May 30, 2018.
