Fig. 2. MgII and the γ-phosphate group of GTP are necessary for high affinity CoII binding.
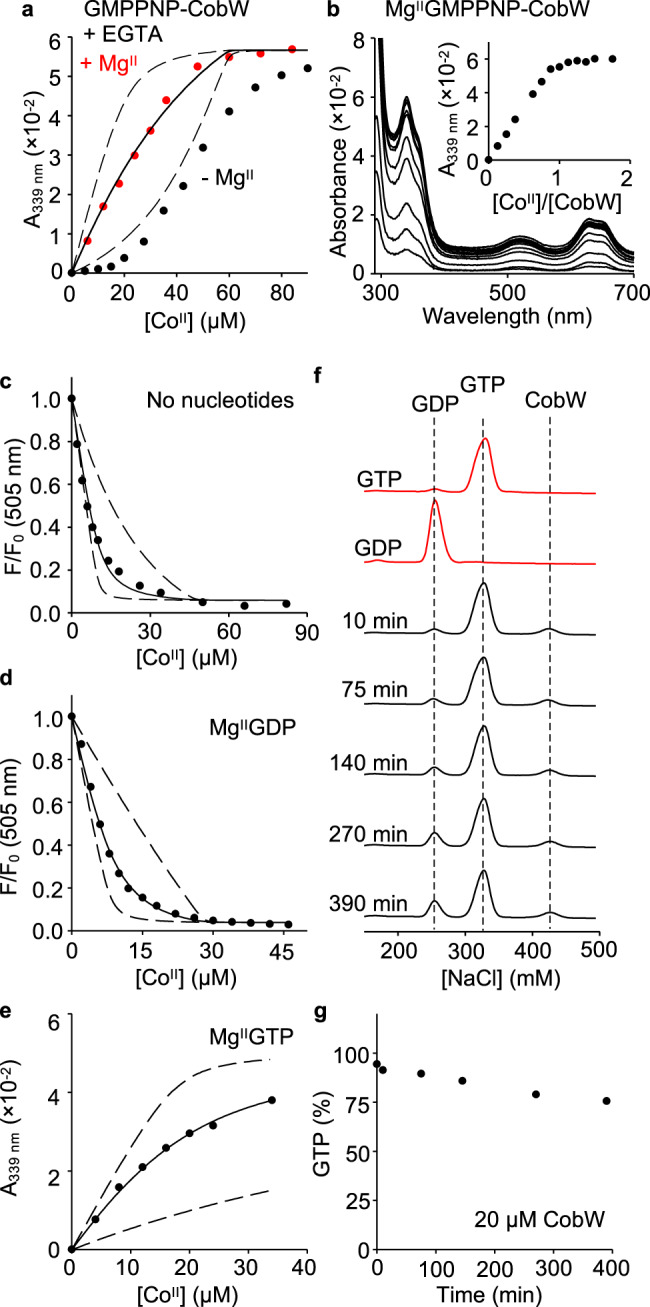
a Absorbance (relative to CoII-free solution) of CoII-titrated CobW (20 µM) with GMPPNP (60 µM) in competition with EGTA (40 µM); titrations in the absence (black) or presence (red) of MgII (2.7 mM, i.e. concentration in a bacterium9,12). Data shown are representative of n = 3 independent experiments (with varying [competitor] and/or identity; see Supplementary Figs. 3c, d and 4a, b). b Absorbance (relative to CoII-free solution) of CoII-titrated CobW (20 µM) with GMPPNP (60 µM) and MgII (2.7 mM) in the absence of competing ligand; feature at 339 nm (inset) showing linear increase saturating at 1:1 ratio CoII:CobW (n = 2; see Supplementary Fig. 3e). c–e Representative KCo(II) quantification for CobW in the absence or presence of nucleotides (n = 3 independent experiments, details in Supplementary Fig. 4 and Supplementary Table 1). c Fluorescence quenching of CoII-titrated fura-2 (10 µM) in competition with CobW alone (37 µM). d Fluorescence quenching of CoII-titrated fura-2 (8.1 µM) in competition with CobW (20 µM) with MgII (2.7 mM) and GDP (200 µM). e Absorbance (relative to CoII-free solution) of CoII-titrated CobW (18 µM) in competition with EGTA (2.0 mM) with MgII (2.7 mM) and GTP (200 µM). Solid traces in a, c, d, e show curve fits of experimental data to a model where CobW binds one molar equivalent CoII per protein monomer. Dashed lines show simulated responses for KCo(II) tenfold tighter or weaker than the fitted value. f Analysis of GTP hydrolysis by anion-exchange chromatography. Control samples of GTP and GDP elute as distinct peaks (red traces) measured by absorbance at 254 nm. Black traces show the extent of hydrolysis of GTP (200 µM) incubated with CobW (20 µM), MgII (2.7 mM) and CoII (18 µM) over time. g Analysis of data from f showing % GTP remaining over time. After 390 min incubation, nucleotides remain primarily (>75 %) unhydrolysed. Equivalent data using 4:1 ratio GTP:CobW is shown in Supplementary Fig. 6.
