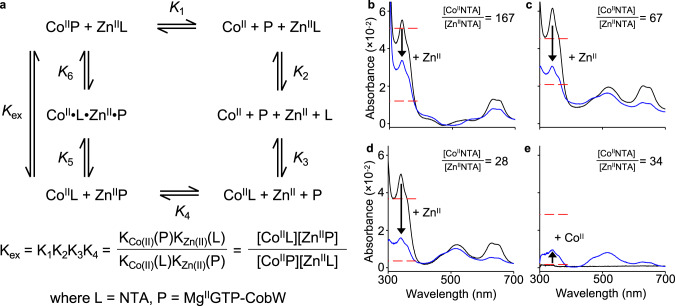
Fig. 4. MgIIGTP-CobW binds ZnII with sub-picomolar affinity.
a Representation of the equilibrium for exchange of CoII and ZnII between ligand (L = NTA) and protein (P = MgIIGTP-CobW). b–e Absorbance (relative to metal-free solution) of solutions of CobW (17.9–20.4 µM), MgII (2.7 mM), GTP (200 µM) and NTA (0.4–4.0 mM) upon (b–d) first addition of CoII (black trace) then ZnII (blue trace) or (e) the reverse, at equilibrium (n = 1 for each panel). The absorbance peak at 339 nm corresponds to CoII-bound protein. An excess of ligand NTA was used to buffer both metals in each experiment: varying the ratios of ligand-bound metal ions ([CoIINTA]/[ZnIINTA] = 28–167) shifted the ratios of CoII- and ZnII-bound protein as predicted by the equilibrium exchange constant in a. Consistent KZn(II) values for MgIIGTP-CobW were generated at all tested conditions (Supplementary Table 4). Dashed red lines show expected A339 nm peak intensities for KZn(II) of MgIIGTP-CobW tenfold tighter or weaker than calculated values.