Figure 5.
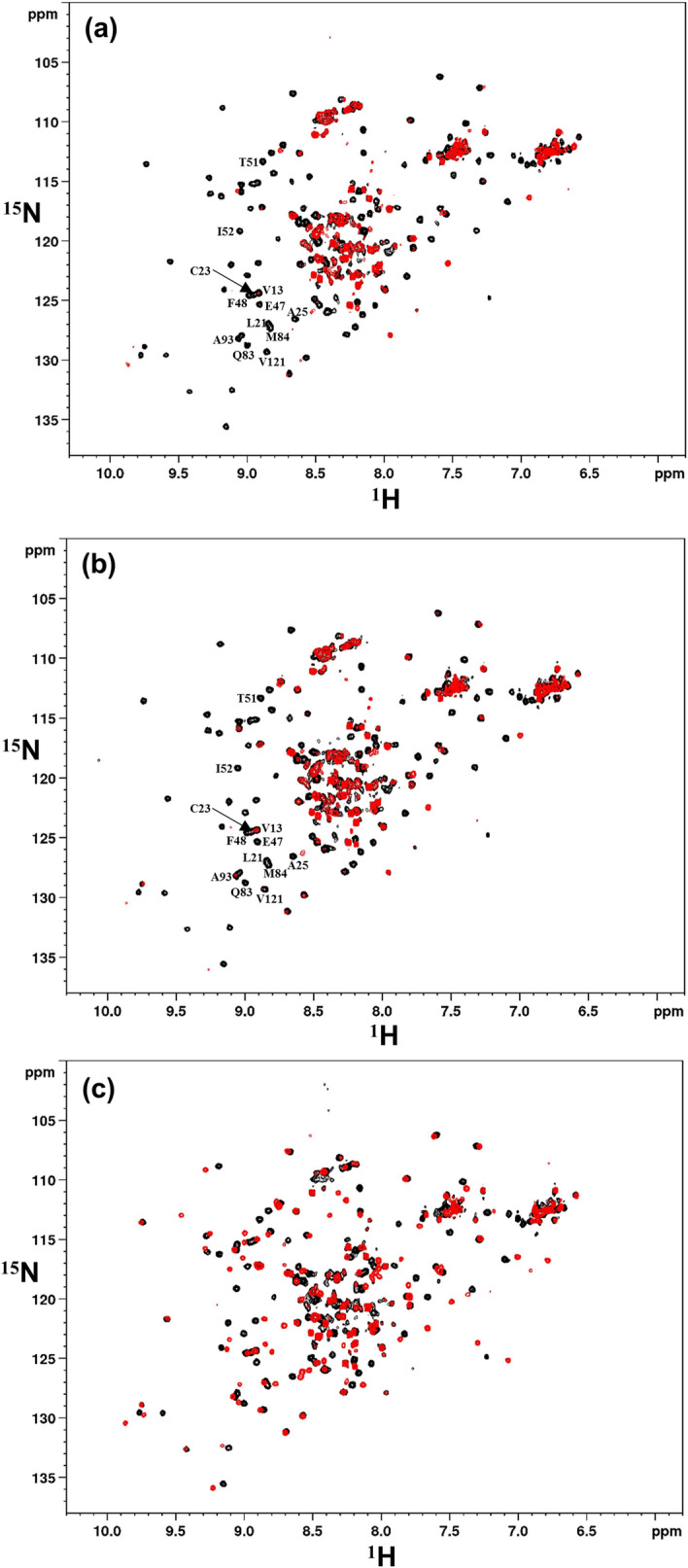
Comparative responses of VHH-IR5 to IGF1R ID fragments. HSQC spectra of 15N-labelled VHH-IR5 identify effects of interactions with three unlabelled IGF1R ID fragments. In black are the HSQC spectra of free VHH-IR5 collected immediately before additions of Ubi-ID, Ubi-s-ID and Pep5 while superimposed in red are the HSQC spectra of the sdAb complexes at ~ 1:1 molar ratio. (a) spectral comparisons showing the effects of Ubi-ID binding. (b) spectra showing the effects of Ubi-s-ID binding. Note that Ubi-ID (a) and Ubi-s-ID (b) binding induce almost the same perturbations, i.e. disappearances of many HSQC signals of 15N-labelled VHH-IR5, especially those of residues T51 and I52 at the beginning of the CDR2 loop and many other residues in the framework region. All perturbed residues were labelled using the resonance assignments of 15N-labelled VHH-IR5 (Fig. S5). (c) Widespread spectral displacements of 15N-labelled VHH-IR5 were produced by binding of the peptide fragment Pep5.
