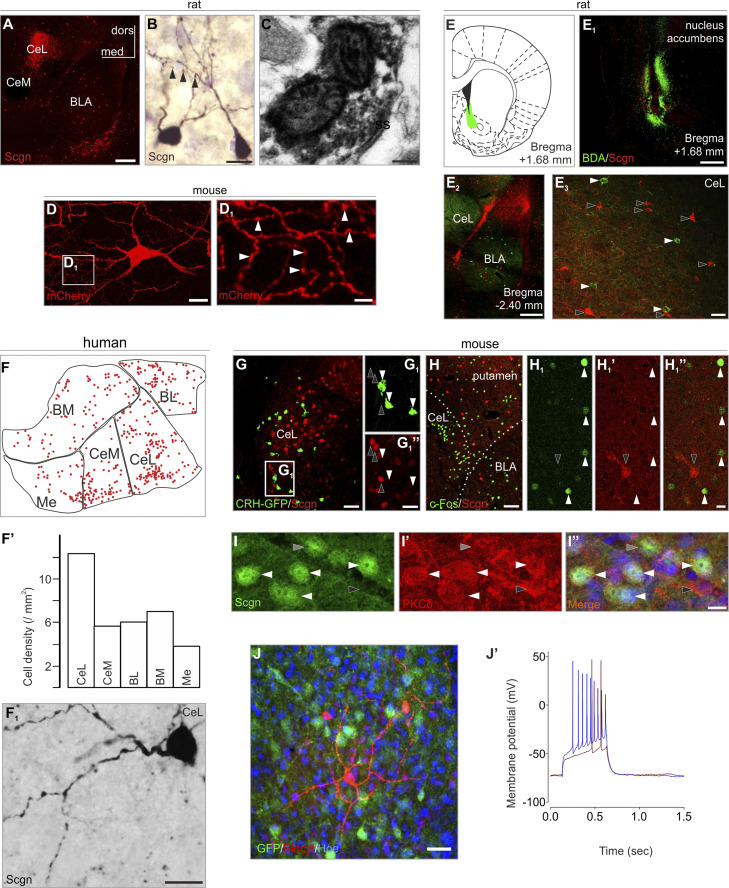
Fig. 1.
Secretagogin (Scgn) labels interneurons in the amygdala. (A) Scgn+ neurons typically populated the CeL (for schemata of representative coronal sections and complete amygdala survey see SI Appendix, Fig. S1). (B) Scgn+ neurons were typically multipolar with spines occasionally observable on their dendrites (arrowheads). (C) Scgn in the presynaptic compartment of a symmetrical synapse. (D and D1) mCherry-labeled interneuron in a Scgn-Cre mouse microinjected with pAAV8-hSyn-DIO-mCherry virus in the CeL. Arrowheads point to varicosities in the local axonal arbour. (E–E3) BDA+ retrogradely labeled neurons (tracer injection: nucleus accumbens) remained Scgn− in the CeL. (F–F1') In human, Scgn+ neurons appeared in highest density in the CeL. (G–G1”) Scgn+ neurons (open arrowheads) and CRH-GFP+ neurons (filled arrowheads) exhibited complementary distribution in the amygdala when using CRH-GFP transgenic mice. (H–H1”) Formalin stress induced c-Fos expression in Scgn− neurons (filled arrowheads). Open arrowhead points to a Scgn+ neuron. (I–I”) Scgn+ neurons typically coexpress PKCδ (white arrowheads point to Scgn+/PKCδ+ neurons; gray and black arrowheads indicate Scgn+/PKCδ− and secretagogin−/PKCδ+ somata, respectively. (J and J’) Whole-cell patch clamp recordings and cell reconstruction in amygdala slices of Scgn-GFP animals. Step current injections produced representative voltage changes in Scgn-GFP+ cells with delayed generation of the first action potential (12 of 13 cells). A and E2 were captured by using the tile-and-stitch function of the ZEN2012 imaging toolbox (Zeiss). BL, basolateral amygdaloid nucleus (human); BM, basomedial amygdaloid nucleus (human); BLA, basolateral amygdaloid nucleus, anterior part (mouse and rat); CeM, central amygdaloid nucleus, medial division (human and rat); CeL, central amygdaloid nucleus, lateral division (human, mouse, and rat); dors, dorsal; med, medial; Me, medial amygdaloid nucleus (human); ss, symmetrical synapse. (Scale bars: A, E1, and E2, 500 µm; C, 200 nm; B, D, F1', H1”, I, and J, 15 µm; D1, 4 µm; E3 and G, 50 µm; G1”, 20 µm; H, 80 µm.)