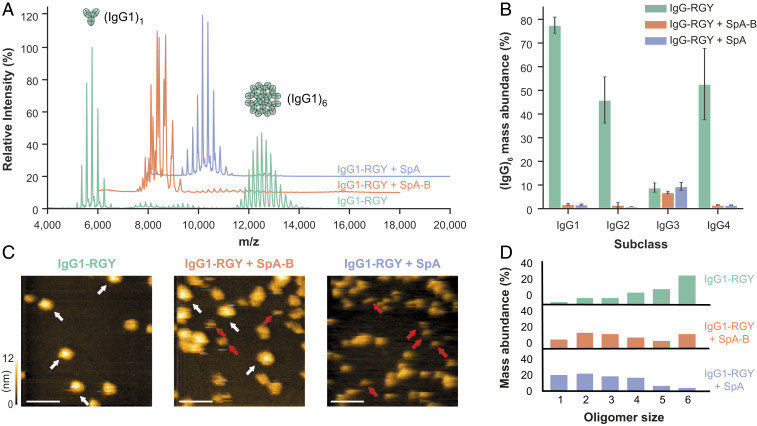
Fig. 3.
Binding of SpA-B or SpA blocks IgG-RGY monomers from assembling into higher-order oligomers. (A) Native mass spectra of IgG1-RGY in the absence (green) and presence of SpA-B (orange) or SpA (blue). (B) Hexamer relative mass abundance of IgG-RGY subclasses in the absence and presence of SpA-B or SpA, assessed by native MS. Error bars indicate the SD over three replicate samples. Although RGY mutations effectively induce hexamer formation of IgG1, IgG2, and IgG4, IgG3-RGY had a lower propensity to form hexamers in solution. (C) HS-AFM images of IgG1-RGY on DNP-SLBs in the absence and presence of SpA-B or SpA, after preincubation in solution. White arrows indicate (IgG1)6; red arrows, (IgG1)1. (Scale bars: 100 nm.) (D) IgG oligomer distribution of IgG1-RGY alone (n = 372) and in the presence of SpA-B (n = 697) or SpA (n = 386) on DNP-SLBs after preincubation in solution. The histogram displays the fraction of IgGs constituting the respective oligomer species. Oligomer distribution was quantified by force-induced dissociation. n refers to the number of individually characterized IgGs. Statistical significance between the three experiments was evaluated with the two-tailed Mann–Whitney U test. Control vs. SpA-B, P < 0.00001; control vs. SpA, P < 0.00001; SpA-B vs. SpA, P < 0.001.