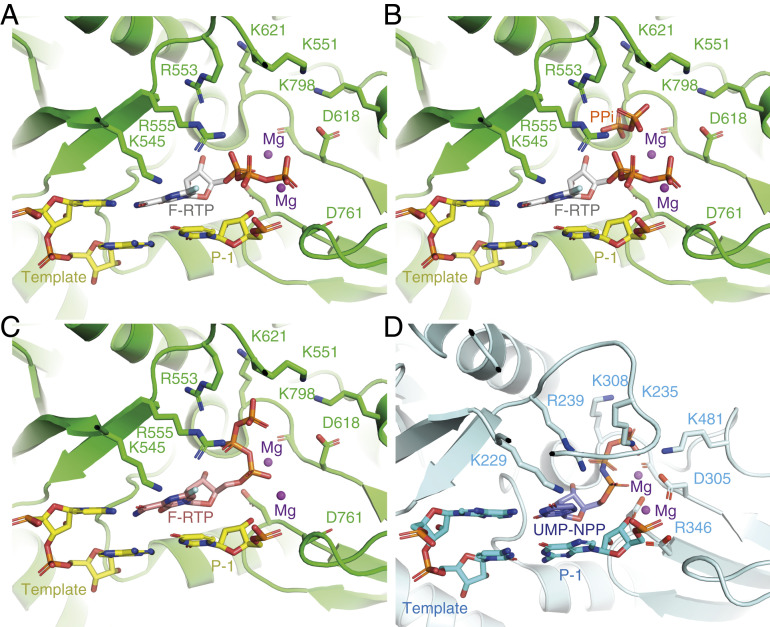
Fig. 4.
Coordination of favipiravir-RTP in the active site of the SARS-CoV-2 RdRp. (A) A nonproductive conformation of favipiravir-RTP (F-RTP), base paired to the P+1 nucleotide of the template strand, as shown, was observed in the cryoEM density map. For clarity, the primer strand is omitted, and only the P1 and P1 nucleotides of the template strand are shown. (B) The nonproductive conformation may be favored in the presence of pyrophosphate (PPi), which is a possible by-product from the incorporation of rNTPs into the RNA. (C) A hypothetical productive conformation of favipiravir-RTP, which may lead to its incorporation into the primer RNA strand, is modeled. (D) For comparison, the position of an incoming nonhydrolyzable UTP analog in the bat influenza polymerase elongation complex is shown (PDB ID code 6SZV) (30).