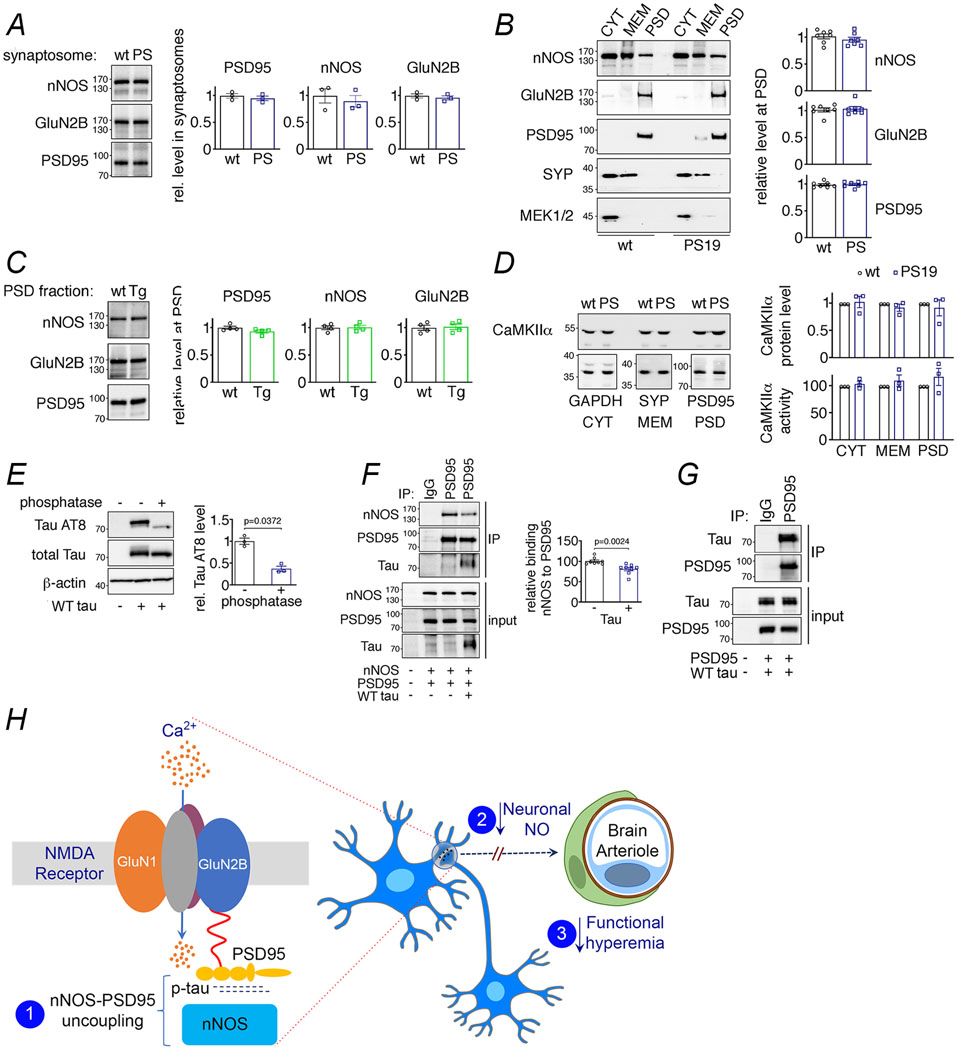
Extended Data Fig. 10. NMDAR-related proteins in PS19 and rTg4510 mice, effects of WT tau on nNOS-PSD95 coupling, and putative mechanisms of the effect of tau on neurovascular function.
A-D. Levels and kinase activity of NMDAR-related proteins are not altered in 2-3 month-old PS19 and rTg4510 mice. A. Protein levels of nNOS, GluN2B and PSD95 are unaltered in synaptosomal preparations of PS19 (PS) compared to WT mice. N=3/group. Data are presented as mean±SEM. B. Protein levels of nNOS, GluN2B and PSD95 in PS19 (PS) and WT mice are comparable. The presynaptic marker synaptophysin (SYP) and MEK1/2 were used as membrane (MEM) and cytosolic (CYT) markers, respectively. N=7/group. Data are presented as mean±SEM. C. As in PS19 mice, nNOS, GluN2B and PSD95 protein levels are unchanged in PSD preparations of rTg4510 (Tg) mice compared to WT mice. N=4/group. Data are presented as mean±SEM. D. CaMKIIα level and activity quantified with reference to GAPDH, synaptophysin (SYP), or PSD95 associated with cytoplasm (CYT), membrane (MEM), and/or PSD are not altered in PS19 mice, compared to WT. N=3/group. Data are presented as mean±SEM. Immunoblots in A-D are cropped; full gel pictures are shown in Source Data 28. E-G. WT tau impairs binding of nNOS to PSD95 through association with PSD95. E. WT tau over-expressed in HEK293T cells is susceptible to phosphatase treatment, and thus hyperphosphorylated. N=3/group; unpaired t-test. F. WT tau co-expression disrupts binding of nNOS to precipitated PSD95. N=8/group; two-tailed unpaired t-test. G. Exogenously expressed WT tau interacts with PSD95 in HEK293T cells. A representative blot from N=3/group is shown. Data are presented as mean±SEM. Immunoblots in E-G are cropped; full gel pictures are shown in Source Data 12. H. Putative mechanism by which tau induces a deficit in neuronal NO and neurovascular dysfunction: pathogenic tau (p-tau) binds to PSD95 and prevents its association with nNOS (1) and the resulting suppression in the NO production evoked by glutamatergic synaptic activity (2) dampens the NO dependent component of the increase in CBF produced by activation (3).