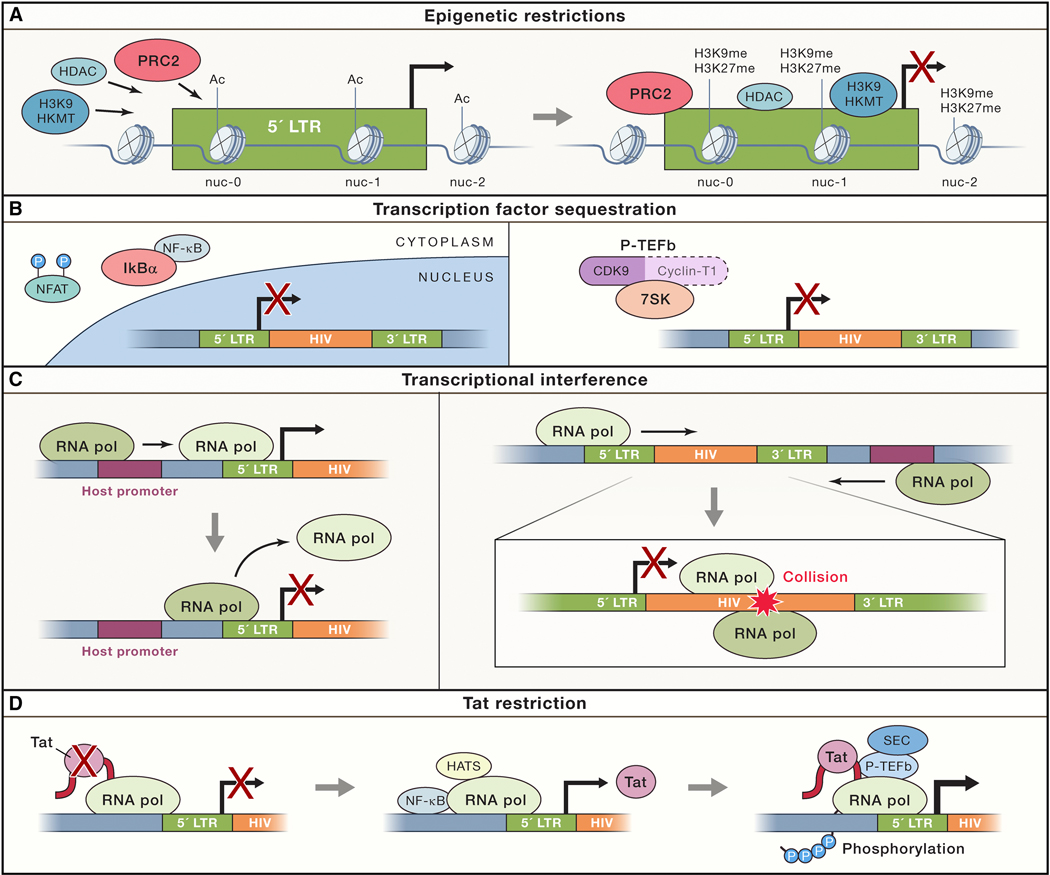
Figure 1.
Proviral silencing and latency is founded and enforced via multiple restrictions to expression. (A) Epigenetic modifiers, such as histone deacetylases (HDACs) and histone lysine methyltransferases (HKMTs), are recruited to HIV-1 LTR promoter, notably by the PRC2 complex. This results in histone modifications within chromatin at the HIV promoter that limit the ability of RNA polymerase to initiate transcription. (B) Sequestration of essential transcription factors like NFAT and NF-kB, and the pTEF-b cyclin complex, are sequestered in resting CD4+ T cells by cellular regulatory complexes (IkB and HEXIM/7skRNA, respectively). (C) Transcriptional interference can occur by promoter occlusion, when a host gene polymerase positioned upstream of the provirus reads through the HIV-1 LTR, causing displacement of necessary transcription factors. Alternatively convergent transcription abort viral expression when the proviral and the host gene RNA Pol II complexes are in opposite orientation, and collide. (D) In the absence of sufficient viral Tat transactivator, viral transcripts are paused. The switch to processive elongation requires the kinase activity of P-TEFb along with the recruitment of processivity factors that constitute the superelongation complex (SEC). Tat efficiently transactivates HIV transcription by recruiting P-TEFb and the SEC to the paused RNAP II complex at the TAR hairpin.