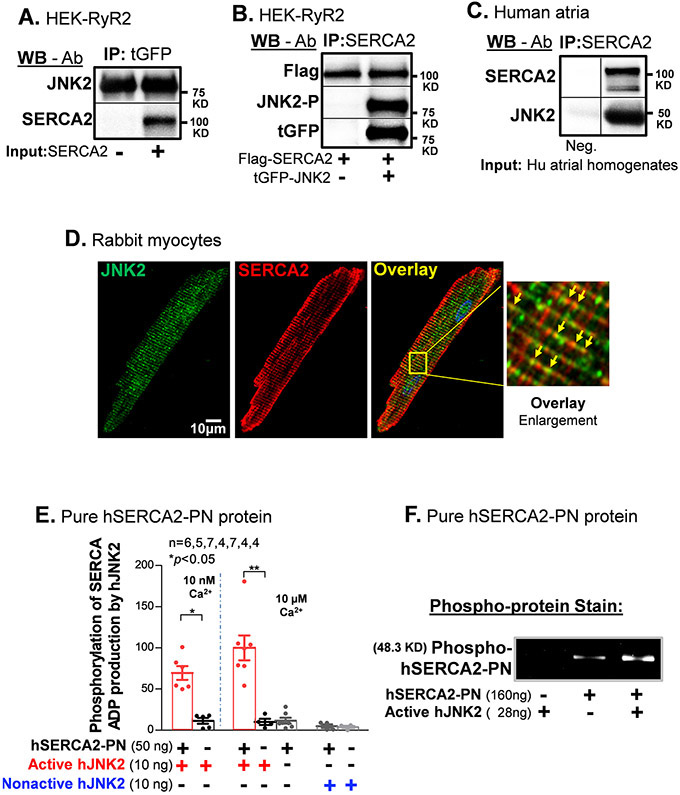
Figure 2.
JNK2 is physically associated with SERCA2 and phosphorylates SERCA2. A) Immunoblotting images of co-immunoprecipitated SERCA2 proteins are associated with pull-down tGFP-JNK2 proteins with a tGFP-specific antibody. B) Immunoblotting images of co-immunoprecipitated JNK2 including phosphorylated JNK2 (JNK-P, detected by phospho-specific JNK antibody) and tGFP-tagged JNK2 (detected by tGFP antibody) with SERCA2-specific antibody pull-down Flag-tagged SERCA2 proteins (Flag-SERCA2, detected by Flag antibody) in HEK cells co-transfected with tGFP-JNK2 and Flag-SERCA2 vectors, while negative control (Flag-SERCA2 alone transfection) is in the left lane. C) Immunoblotting images of co-immunoprecipitated SERCA2 proteins with a SERCA2-specific antibody are associated with pull-down JNK2 proteins in human atrial homogenates, while negative control (no SERCA2 antibody) is in the left lane. D) Double immunofluorescence staining of JNK2 and SERCA2 antibodies in rabbit myocytes showing a typical striation pattern of SR for both JNK2 (green) and SERCA2 (red). Far right panel shows an enlarged image of well-colocalized JNK2 and SERCA2 signals (yellow arrows) distributing in a striation pattern. E) Summarized data of increased ADP production of pure active-hJNK2 (but not nonactive-hJNK2) phosphorylation of hSERCA2-PN proteins, with either 10 nM Ca2+ (left panel) or 10 μM Ca2+ (right panel), while the level of ADP production was minimal in the baseline controls of either hJNK2 alone and SERCA alone. F) A Pro-Q Diamond staining gel image of phosphorylated SERCA2-PN proteins (160 ng) by active-hJNK2 (far-right lane) and baseline phosphor-signals of SERCA2-PN alone (middle lane), and undetectable signal of active-hJNK2 alone (far-left lane).