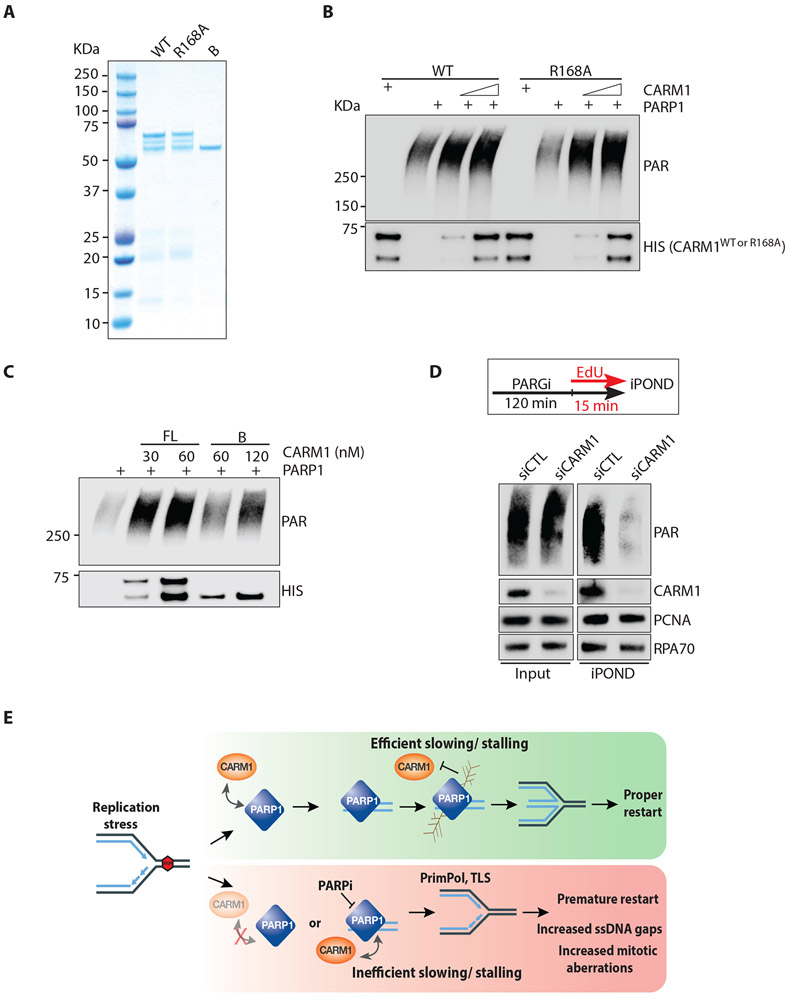
Figure 6. CARM1 stimulates PARP1 in vitro and promotes PARylation at replication forks.
(A) Purified proteins (300 ng) of full-length, wild-type CARM1WT (WT), CARM1R168A (R168A), and truncated CARM1B (B) were resolved by SDS-PAGE and stained with Coomassie blue. (B) In vitro PARP1 activation assay with purified PARP1 (0.5 nM), NAD+ (250 μM), and calf thymus dsDNA (25 μg/mL). As indicated, increasing concentrations (17-34 nM) of CARM1WT (WT) or CARM1R168A (R168A) were added to reactions. Levels of PAR and CARM1 variants were analyzed by Western blot with the indicated antibodies. (C) In vitro PARP1 activation assay was performed and analyzed as in (B). CARM1WT and CARM1B were added to reactions at the indicated concentrations. (D) HEK293T were transfected with control or CARM1 siRNA for 48 h. Cells were treated with 1 μM PARGi (PDD00017273) for 2 h 15 min, and were labeled with EdU during the last 15 min. Proteins at progressing forks were captured by iPOND, and levels of indicated proteins were analyzed by Western blot. (E) A model depicting how CARM1 and PARP1 control replication fork speed and the choice of stress response mechanisms.