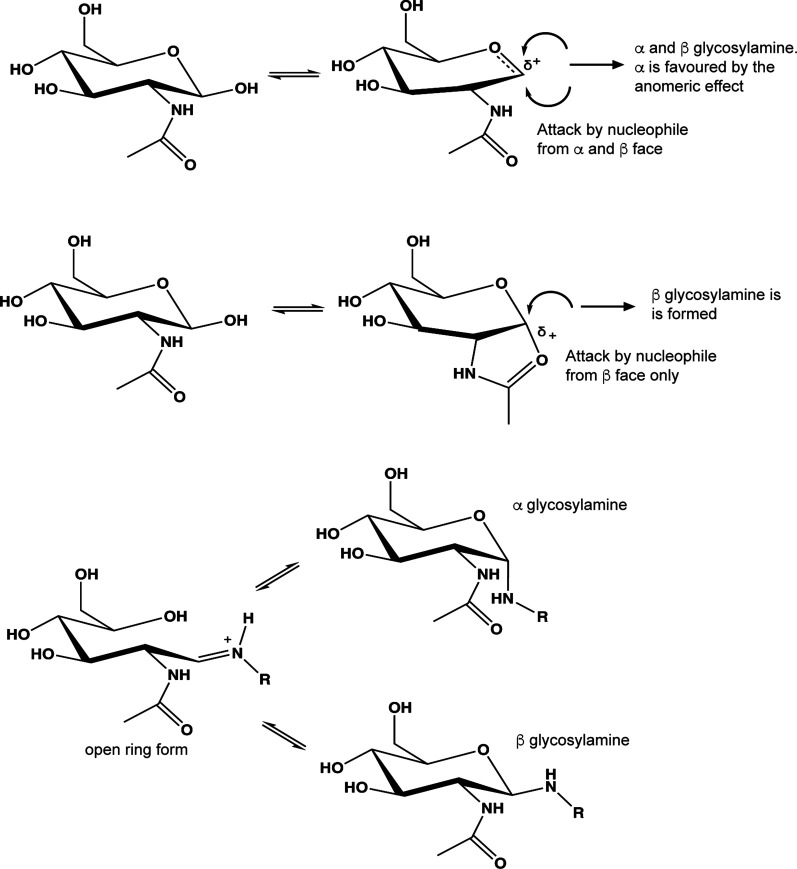
Figure 4. Labelling mechanism and anomeric configuration of products.
The observed (∼1 : 1) mixture of the glycosylamine products of the reaction between 2-amino benzoic acid and N-acetylated d-hexosamines can be envisaged as arising from two possible mechanisms; (A) Proceeding via an oxocarbenium ion intermediate produces both possible anomers, but the α form is expected to dominate owing to the anomeric effect, although this is a weaker effect for nitrogen than for oxygen; (B) Proceeding through an oxazoline intermediate resulting exclusively in the β anomer; (C) The glycosylamine product, however, may be subject to mutarotation via an open chain form allowing equilibration of the α and β anomers. R = BODIPY-FL hydrazide or 2-amino benzoic acid (2-AA).