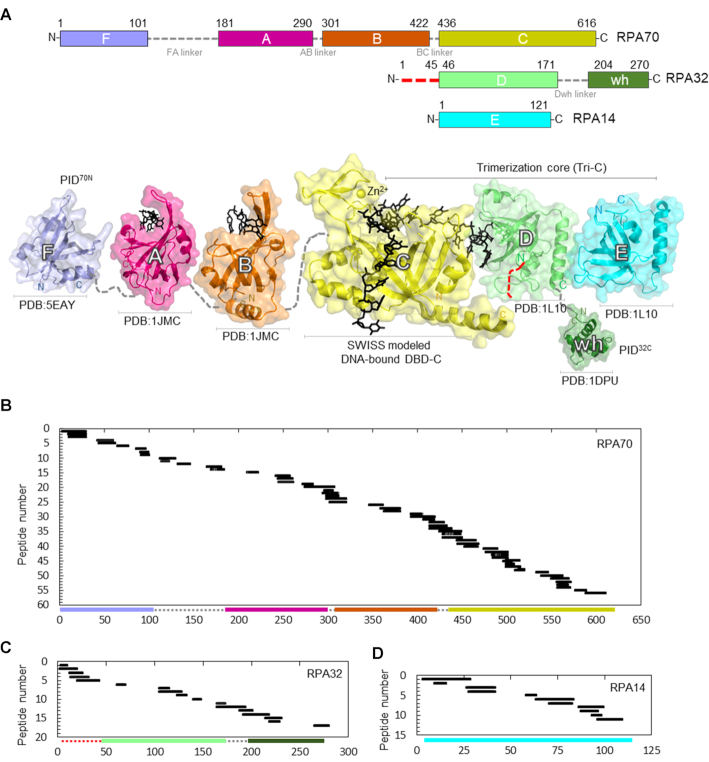
Figure 1.
Sequence coverage of the DNA binding and protein interaction domains of RPA. (A) Top: Domain composition of human RPA70, RPA32 and RPA14 subunits and their respective linkers. The red dotted line in the N-terminus of RPA32 denotes the region of phosphorylation. Bottom: The crystal structures of the individual domains of RPA are depicted and color coded to match the domains on the top panel. For domain C, DNA is modeled onto DBD-C using SWISS-MODEL with the Ustilago maydis structure as reference. Peptides identified in the MS analysis corresponding to (B) RPA70, (C) RPA32 and (D) RPA14 are shown. 56 peptides from RPA70 (59% sequence coverage), 17 peptides from RPA32 (50% sequence coverage) and 11 peptides from RPA14 (49% sequence coverage) were identified. The y-axis indicates the peptide fragment number, and the x-axis denotes peptides fragments arranged in order of increasing residue numbers from the N-terminus of each subunit as denoted in Supplementary Table S1.