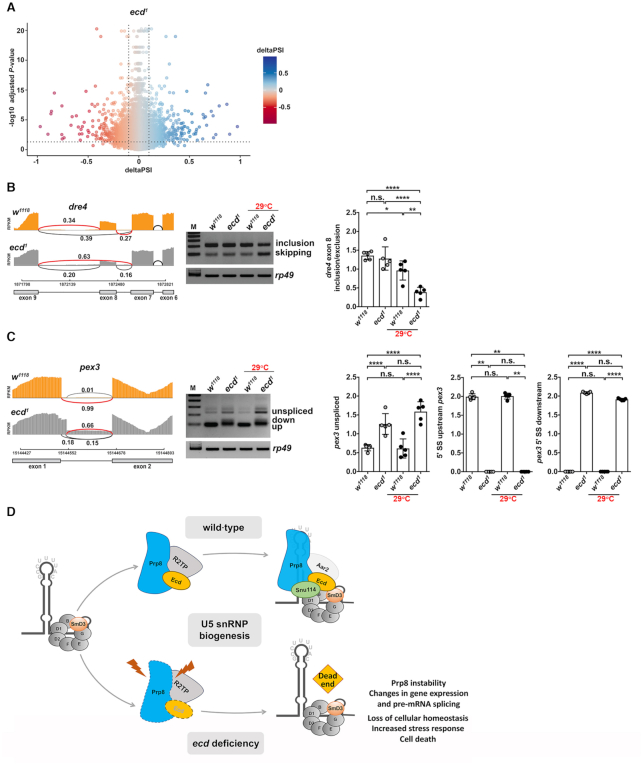
Figure 8.
Ecd deficiency causes global alterations in splicing pattern. (A) The volcano plot shows changes in splicing pattern in ecd1 homozygous mutant third instar larvae upshifted to 29°C versus control (w1118) identified by LeafCutter. Every dot represents a splicing event. Dotted lines demarcate significantly changed events with delta percent spliced in deltaPSI ≥ 0.1 and adjusted P-value ≤ 0.05. (B, C) Sashimi-plots of selected candidate genes dre4 (B) and pex3 (C) from mRNA-seq data show alterations of splicing patterns in ecd1 homozygous mutant larvae compared to control (w1118). The representative images of semiQ-PCR gels and quantifications confirm missplicing events in dre4 (B) and pex3 (C) in ecd1 homozygous mutant wing discs relative to control dissected from third instar larvae that were kept at 18°C or upshifted to non-permissive temperature (29°C). Note that alteration to pex3 pre-mRNA splicing is already apparent in ecd1 samples at 18°C. Levels of rp49 transcripts were used for normalization. Data represent means ± SD, n = 5. Two-way ANOVA with Tukey's multiple comparison test was used to determine significance, *P < 0.05, **P < 0.01, ****P < 0.0001, n.s. = non-significant. (D) Proposed model for the role of Ecd in the cytoplasmic part of U5 snRNP biogenesis. U5 snRNP maturation is a step-wise process that starts in cytoplasm with SMN-guided assembly of the heptameric Sm protein ring around the conserved Sm binding site on the U5 snRNA. The generated core U5 snRNP particle then serves as a platform for association of further U5 snRNP-specific proteins. Ecd functions as a chaperon that, through interaction with SmD3, brings Prp8. The effective U5 snRNP biogenesis relies on binding cooperativity of Ecd and Prp8 and interactions with U5 snRNA. In the absence of Ecd, the core U5 snRNP particle forms properly, but Prp8 protein remains unshielded and destabilized, which interferes with U5 snRNP maturation, ultimately leading to spliceosome scarcity, transcriptome-wide splicing errors and cell death. See also Supplementary Figure S7 and Supplementary Dataset S4.