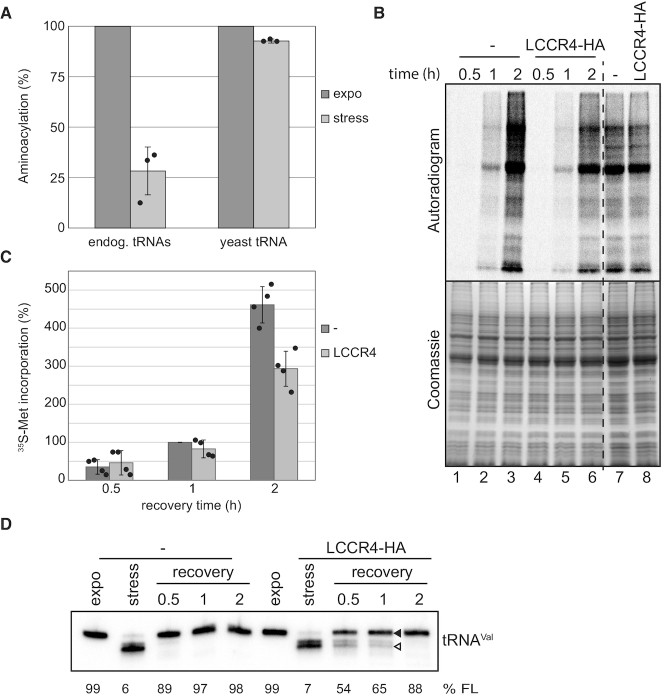
Figure 7.
Repairing of shortened tRNAs is crucial for translation during stress recovery. (A) In vitro aminoacylation was tested on S100 cell fractions from cells grown exponentially or stressed for 2 h in PBS, using as substrate either the endogenous tRNAs (endog. tRNAs) or after supplementation with total yeast tRNA (yeast tRNA). Dark and light grey bars correspond to signals from exponential or stressed samples, respectively. The percentage of aminoacylation was calculated by normalization to the signal for S100 from exponentially growing cells. Depicted is the mean of three experiments ± SD. (B) Cells induced for LCCR4-HA overexpression or not were stressed and translation efficiency investigated during stress recovery by metabolic labeling with 35S-Methionine. After the indicated times cells were pelleted, proteins separated by SDS-PAGE and incorporated radioactivity detected by exposing to phosphorimager screens. Unstressed cells labeled for 30min were also tested (two most right lanes). The upper panel shows a representative autoradiogram of the metabolic labeling experiment while the lower panel is the corresponding gel stained with coomassie. (C) Quantification of 35S-Methionine incorporation of 4 independent experiments. Dark and light grey bars correspond to the percentage of 35S-incorporation in uninduced and induced cells, respectively. Signals were normalized to the intensity corresponding to uninduced cells recovered for 1h. Data are represented as mean ± SD. (D) RNA was extracted from cells treated as in (B) and tRNA repair was investigated by northern blot against tRNAVal. The percentage of full length tRNAVal present is indicated (%FL).