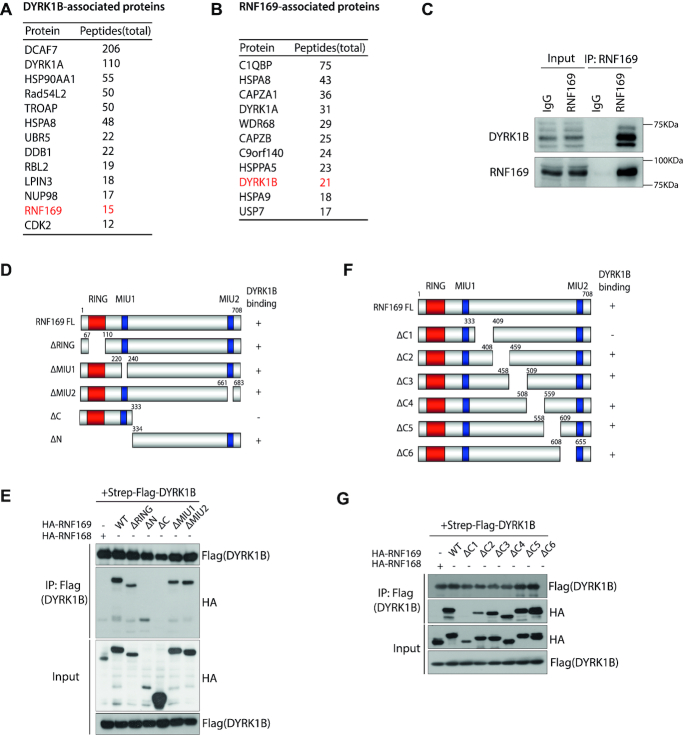
Figure 3.
RNF169 is a bona fide DYRK1B-interacting protein. (A) Affinity purification of DYRK1B complex identified a list of DYRK1B-interacting proteins. Names and total peptides of the top hits are listed. RNF169 is highlighted in red. (B) RNF169-associated proteins were identified from tandem affinity purification of RNF169 protein. DYRK1B is shown in red. (C) Co-immunoprecipitation (IP) in HEK293T cells. Whole cell lysates incubated with protein A beads were supplemented with either IgG antibody or RNF169 antibody at 4°C overnight. Protein A-conjugated proteins were subjected to western blotting using anti-DYRK1B and anti-RNF169 antibodies. (D) Schematic illustration of RNF169 protein domains. ‘+’ represents DYRK1B binds to the indicated RNF169 truncated mutants and ‘–’ denotes DYRK1B binding defective mutants. NLS, nuclear localization signal; RING, really interesting new gene; MIU, motif interacting with ubiquitin. (E) HEK293T cells were transiently co-transfected with Strep-Flag tagged DYRK1B and HA tagged (HA-) wildtype (WT) RNF169 or the indicated RNF169-truncated mutants. Cell lysates were incubated with Streptavidin beads and the immunoprecipitates were subjected to western blotting using anti-Flag and anti-HA antibodies. HA tagged RNF168 (HA-RNF168) was used as a DYRK1B-binding defective control. (F) Graph depicting the C-terminal deletion mutants of RNF169 protein. ‘+’ indicates DYRK1B-binding mutants and ‘−’ denotes DYRK1B-binding defective mutants, respectively. (G) HEK293T cells were co-transfected with Strep-Flag tagged DYRK1B and HA tagged RNF169 WT or RNF169 C-terminal deletion mutants. After lysis, the lysates were processed for co-immunoprecipitation using the indicated antibodies.