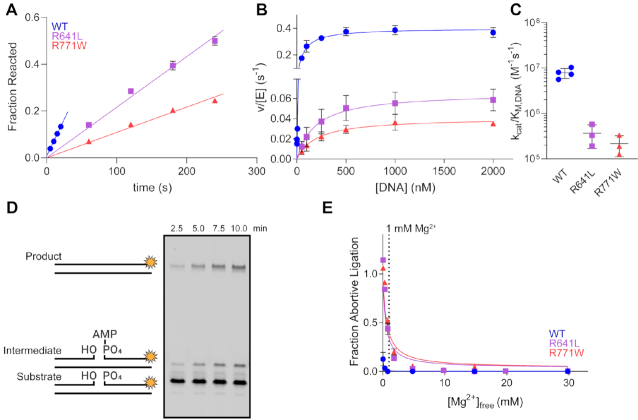
Figure 4.
LIG1 syndrome mutations decrease catalytic efficiency and increase abortive ligation. (A) Representative time course from multiple turnover reactions containing 10 nM enzyme, 500 nM DNA, 2 mM MgCl2 (1 mM Mg2+free) and 1 mM ATP. Initial rates from multiple turnover reactions were determined from the slope of linear fits to the amount of substrate reacted. (B) The steady-state DNA dependence was determined with 0.1–10 nM LIG1 and increasing amounts of the DNA substrate. The resulting data were fit by the Michaelis–Menten equation to obtain kcat and KM,DNA values for WT and mutant LIG1 enzymes. (C) The R641L and R771W mutants have substantially reduced catalytic efficiency (kcat/KM values from the fits in panel b). (D) Representative denaturing gel image from a ligation assay containing 5 nM R641L, 500 nM DNA, 2 mM MgCl2 (1 mM Mg2+free) and 1 mM ATP highlighting buildup of the aborted intermediate. (E) The fraction of abortive ligation events observed in the steady-state magnesium dependences. The physiologically-relevant [Mg2+]free (∼1 mM) is marked by a dashed line. R641L and R771W LIG1 exhibit enhanced rates of abortive ligation compared to WT at low [Mg2+]free. All values are mean ± SD (n ≥ 3).