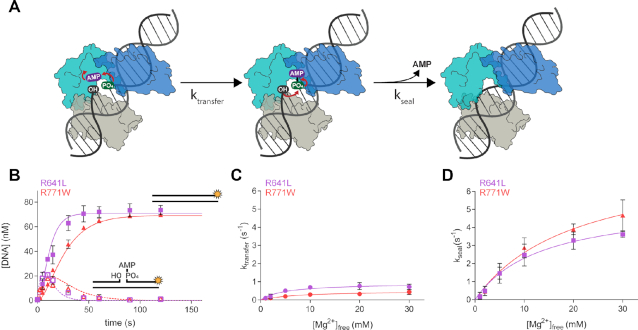
Figure 5.
LIG1 clinical mutations weaken catalytic metal affinity in both the adenylyl transfer and nick-sealing steps. (A) After the adenylylated LIG1 enzyme binds to a nick site, the AMP moiety is transferred to the 5′ phosphate during a step known as adenylyl transfer. Nick-sealing follows with attack of the 3′ hydroxyl on the 5′ phosphate, releasing AMP and the sealed DNA product. (B) Representative single-turnover ligation time courses for R641L (lavender) and R771W (red) in the presence of 1 mM Mg2+. Data were fit to a two-step irreversible model in Berkeley-Madonna to determine the microscopic rates of ligation. The rates of adenylyl transfer (C) and nick sealing (D) were determined from single-turnover experiments containing 800 nM LIG1, 80 nM DNA and varying concentration of Mg2+. The maximal rates and KMg values for both steps were determined from hyperbolic fits to the single-turnover data and the values are summarized in Table 2. All values are reported as the mean ± S.D. (n ≥ 3).