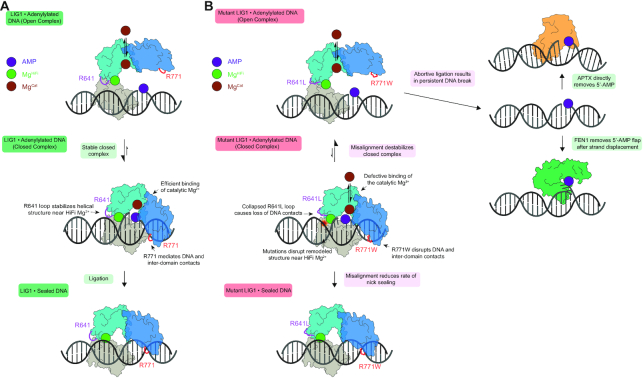
Figure 7.
Cooperative engagement of DNA and impact of LIG1 syndrome mutations on ligase efficiency and abortive ligation. (A) LIG1 encircles a DNA nick, remodeling the DNA substrate and forming a network of DNA binding interactions and interdomain contacts. Tight binding of the adenylylated intermediate (central complex) prevents premature release via an open complex (top) and ensures high efficiency ligation (bottom). (B) The R641L and the R771W mutations disrupt DNA-binding interactions and remodel the DNA-binding surface and interdomain contacts. This destabilizes the closed, high affinity complex as demonstrated by weaker Mg2+ binding and increased frequency of abortive ligation (top). These defects can be overcome by saturation of the active site Mg2+ cofactor (bottom). Abortive ligation, which is a shared feature of the LIG1 syndrome variants, may contribute to the deleterious cellular effects which may be further modulated by pathways for repair of adenylylated DNA, including strand-displacement and flap cleavage by FEN1 or direct hydrolytic removal by APTX.