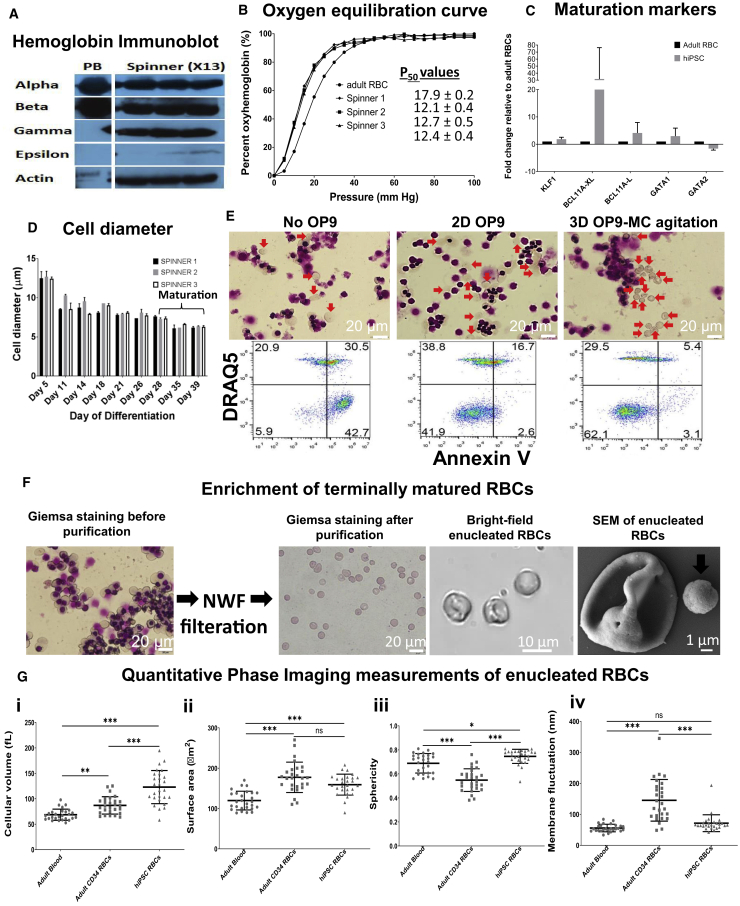
Figure 5.
Terminal Maturation of X13 hiPSC-Derived Erythroblasts from Spinner Differentiation
(A) Cell lysates from peripheral blood (PB) and X13 hiPSC-derived erythroblasts from spinner differentiation (day 27 post differentiation) were immunoblotted with antibodies specific to alpha, beta, gamma, and epsilon human hemoglobin subtypes and the housekeeping control human β-actin. White lines demarcate regions of gel images that were merged.
(B) Oxygen equilibrium curves (percentage oxyhemoglobin versus oxygen pressure [mm Hg]) for adult RBCs (•) and erythroblasts differentiated from X13 hiPSCs (day 27 post differentiation) from spinners 1 (▪), 2 (▲), and 3 (▼) are shown.
(C) qRT-PCR quantified fold expression (GAPDH-normalized and relative to adult erythroblasts) of genes involved in erythroid maturation from X13 hiPSC-derived erythroblasts (day 27 post differentiation) terminally matured for 11 days (data represent the mean ± SD of at least three independent replicates). Representative experiment shown.
(D) Change in cell diameter during differentiation in three independent spinner flask experiments. Day 28 erythroblasts were further matured until day 39.
(E) (Top) Giemsa staining and (bottom) flow cytometry evaluation for non-apoptotic (Annexin V−) enucleated RBCs (DRAQ5−) of X13 hiPSC erythroblasts after 21 days of terminal maturation without OP9 co-culture (No OP9), with monolayer OP9 co-culture (2D OP9), and with OP9-MC co-culture under agitation (3D OP9-MC). Red arrows indicate enucleated erythroid cells. Representative data from n = 3 independent experiments.
(F) Giemsa staining of terminally matured erythroblasts before and after processing through NWF filters is shown. Scale bar, 20 μm. Bright-field images highlighting enucleated RBCs with biconcave appearance and SEM image of a single enucleated RBC are shown. Scale bar, 1 μm. Arrows indicate the expelled pyrenocyte.
(G) Quantitative phase imaging data (mean ± SD) for (i) cell volume, hiPSC RBCs were significantly greater compared with adult CD34 and adult blood; (ii) surface area, hiPSC RBCs were significantly higher compared with adult blood; (iii) sphericity, hiPSC RBCs were significantly greater compared with adult CD34 (∗∗∗p < 0.0001) and adult blood (∗p < 0.05); and (iv) refractive index tomography measurements for dynamic membrane fluctuations of RBCs, hiPSC RBCs were significantly lower compared with adult CD34 RBCs (∗∗p < 0.001). RBCs were differentiated from adult CD34 cells (n = 27 cell counts) and hiPSCs (n = 27 cell counts) and adult blood (n = 27 cell counts).