Figure 4.
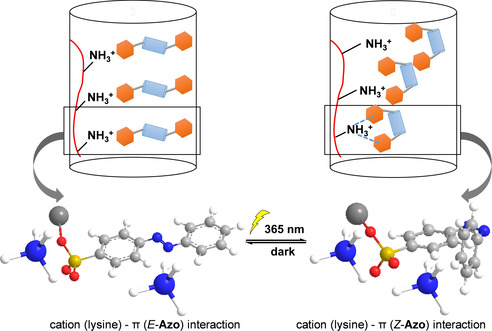
Schematic representation of a plausible mechanism for SUP‐Azo fibers with photo‐switchable mechanical properties. The geometrical rearrangement of the azobenzene units during photo‐isomerization effectively contributed to alterations of the interactions between azobenzene phenyl rings and unoccupied cationic lysine residues of neighboring SUP‐Azo complexes. Therefore, the mechanical properties of SUP‐Azo fibers might be tuned by light. (The blue/white spheres represent NH4 +, the red curve represents the SUP backbone, the red/yellow spheres represent SO3 −, the orange hexagon represents the phenyl rings of azobenzene, and the light blue cylinder represents the N=N bond). The geometrical rearrangement of the azobenzene units during the isomerization process, effectively altering the distance between the external azobenzene phenyl ring and unoccupied cationic lysine residues of neighboring SUP‐Azo complexes. The isomerization from E‐ to Z‐configuration decreases the distance between the two azobenzene phenyl rings, thereby strengthening the cation‐π interactions since in the Z‐isomer both phenyl rings can interact with the protonated ϵ‐amino group of lysine. As a result, this change might improve the fiber's mechanics (e.g. tensile strength, Young's modulus, and toughness) since the cohesion forces within the material are strengthened.
