Figure 3.
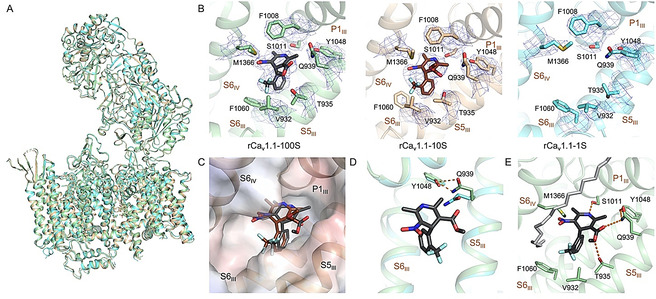
The conformation of nanodisc‐embedded rCav1.1 remains nearly unchanged with SBK applied at different conformations. A) Structural comparison of rCav1.1‐100S (green), 10S (gold), and 1S (cyan). Despite different concentrations of SBK applied, the overall structure remains nearly unchanged. B) There is no density of SBK in the EM reconstruction of rCav1.1‐1S. The densities for SBK and its surrounding residues, all contoured at 7σ in PyMol, are shown as blue mesh. C) Slight displacement of SBK in rCav1.1‐100S (black) and 10S (brown). The electrostatic surface potential of rCav1.1‐100S, in a semi‐transparent presentation, was calculated in PyMol. D) Gln939 on S5III moves toward Tyr1048 in the presence of SBK, as seen between rCav1.1‐100S (green) and rCav1.1‐1S (cyan). This local shift is important for accommodating the ligand. E) Coordination of SBK by both polar and hydrophobic residues. Potential H bonds are shown as dashed lines. A nearby lipid (gray sticks) that blocks the III–IV fenestration with one hydrophobic tail was resolved.
